This article has been
cited by other articles in ScienceCentral.
Abstract
Anaphylaxis to polyethylene glycol (PEG) is rare and mainly occurs with the use of laxatives containing PEG. Recently, an increasing number of PEG allergies have been reported, particularly those related to coronavirus disease 2019 (COVID-19) vaccines. mRNA COVID-19 vaccines, such as the BNT162b2 (Pfizer–BioNTech) and mRNA-1273 (Moderna) vaccines, contain PEG2000 as an excipient and are contraindicated when allergy to a vaccine component exist. We report a 55-year-old woman’s history as a case of successful mRNA COVID-19 vaccination and colonoscopy after oral desensitization to PEG in a patient with PEG allergy who required both COVID-19 vaccination and colon evaluation. Allergy to PEG was diagnosed based on clinical history, skin test results, and basophil histamine release testing. Oral desensitization effectively suppressed histamine release from basophils in response to PEG stimulation, suggesting that oral desensitization using PEG-based laxatives may be an effective treatment option for patients with allergy to the substance.
Go to :

Graphical Abstract
Go to :

Keywords: Allergy, Anaphylaxis, Colonoscopy, Coronavirus disease 2019, Polyethylene Glycol, Vaccine
INTRODUCTION
Polyethylene glycol (PEG) is widely used as an additive in several pharmaceuticals and cosmetics. PEG with a molecular mass of 3,350 g/mol (PEG3350) is also used as a colon cleansing agent due to its low absorption rate in the gastrointestinal tract.
12
Anaphylaxis or allergic reactions to PEG are rare and mainly occur in relation to laxative use, with less than 100 cases reported. However, PEG allergies have increasingly been reported recently, particularly in relation to coronavirus disease 2019 (COVID-19) vaccines.
345 mRNA COVID-19 vaccines, such as the BNT162b2 (Pfizer–BioNTech) and mRNA-1273 (Moderna) vaccines, contain PEG2000 as an excipient, making post-vaccine allergic reactions a problem in patients with PEG allergy.
6 In contrast, virus vector-based vaccines, including the ChAdOx1 (Oxford-Astrazeneca) and Ad26.COV2.S (Johnson & Johnson) vaccines, contain polysorbate 80 (PS80). Vaccines for COVID-19 are contraindicated when an allergy to one of the vaccine components exists. Therefore, patients with PEG allergy may alternatively receive vector-based vaccines.
678
We reported a case of successful mRNA COVID-19 vaccination and colonoscopy after oral desensitization to PEG in a patient with PEG allergy who required vaccination and colon evaluation. This is the first report of mRNA COVID-19 vaccination following oral desensitization to PEG.
Go to :

CASE DESCRIPTION
A 55-year-old woman with a history of gastric ulcer visited our allergy clinic in 2017 with systemic urticaria, throat swelling, and dyspnea 10–30 minutes after taking laxatives for a colonoscopy. She had undergone a colonoscopy without complications in 2007 but, in 2012, developed a feeling of tongue numbness and generalized urticaria after drinking a cup of Colyte-F Powder® (Taejoon Pharm Co., Seoul, Korea) for a colonoscopy. She experienced the same symptoms after taking two different laxative brands and had to visit an emergency room due to generalized rash and dyspnea when the last symptom had occurred a year prior. She also had a history of developing urticaria twice after taking cold medication, but the exact name of the drugs could not be obtained. One month prior to visiting our clinic in search of a safe colonoscopy method, she experienced urticaria one hour after taking a vitamin B preparation (B-max®, Hanpoong Pharm & Foods Co., Seoul, Korea) containing PEG6000.
Her initial vital signs and laboratory test results were within normal range, total serum immunoglobulin E (IgE) level was 189 IU/mL, and skin prick tests (SPTs) to common allergens showed a negative response.
The three laxatives that caused adverse reactions were all identified as PEG3350, and similar symptoms occurred after taking vitamin supplements containing PEG6000. SPTs to 10 mg/mL and 100 mg/mL of PEG were negative, but intradermal test (IDT) showed a positive reaction to 1 mg/mL of PEG (saline, 0 × 0 mm; histamine control, 6 × 4/25 × 20 mm; PEG, 5 × 4/20 × 18 mm). The patient was diagnosed with PEG allergy. Although colonoscopy could be performed using cleansing agents other than PEG, laxatives such as sodium picosulfate were not suitable for this patient due to gastric ulcer. After detailed discussion with the patient, desensitization to PEG was attempted.
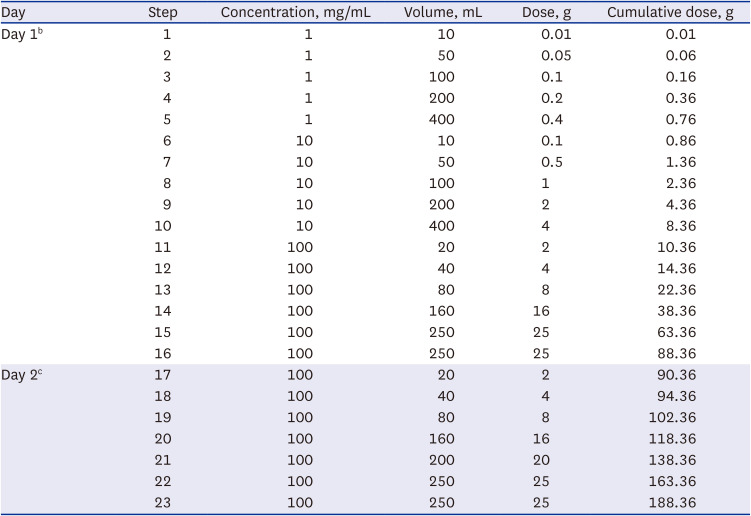
Oral desensitization to PEG was performed in a two-day, 23-step protocol using serial 10-fold dilutions of PEG3350 solution (Coolprep Powder
®, Taejoon Pharm Co.) (
Table 1). Prior to desensitization, hydrocortisone and chlorpheniramine were administered. The desensitization process was completed without symptoms and the colonoscopy was safely performed. No abnormal findings were observed. Immediately prior to and following desensitization, we conducted basophil histamine release (HR) tests using the previously reported method.
9 HR testing showed that PEG induced specific HR only from the patient’s basophils, and that HR was significantly decreased after desensitization (
Fig. 1). The patient was instructed to avoid laxatives or other drugs containing PEG.
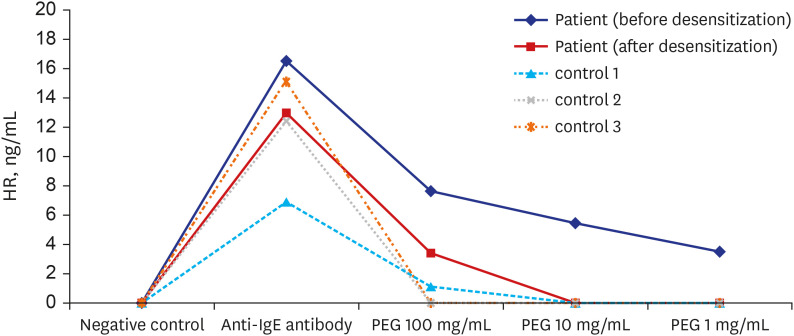 | Fig. 1
Basophil HR test in response to PEG. Peripheral blood collected with leukocyte suspension from the patient and three healthy controls without allergy to PEG. After incubation with stimulants, including three concentrations of PEG3350 and anti-human IgE antibody (KPL, Gaithersburg, MD, USA), released histamine is quantified by LC–MS/MS method. Results from HR tests performed immediately before and after PEG desensitization in the patient are shown.
HR = histamine release, PEG = polyethylene glycol.

|
Table 1
Polyethylene glycol oral desensitization protocola
|
Day |
Step |
Concentration, mg/mL |
Volume, mL |
Dose, g |
Cumulative dose, g |
|
Day 1b
|
1 |
1 |
10 |
0.01 |
0.01 |
|
2 |
1 |
50 |
0.05 |
0.06 |
|
3 |
1 |
100 |
0.1 |
0.16 |
|
4 |
1 |
200 |
0.2 |
0.36 |
|
5 |
1 |
400 |
0.4 |
0.76 |
|
6 |
10 |
10 |
0.1 |
0.86 |
|
7 |
10 |
50 |
0.5 |
1.36 |
|
8 |
10 |
100 |
1 |
2.36 |
|
9 |
10 |
200 |
2 |
4.36 |
|
10 |
10 |
400 |
4 |
8.36 |
|
11 |
100 |
20 |
2 |
10.36 |
|
12 |
100 |
40 |
4 |
14.36 |
|
13 |
100 |
80 |
8 |
22.36 |
|
14 |
100 |
160 |
16 |
38.36 |
|
15 |
100 |
250 |
25 |
63.36 |
|
16 |
100 |
250 |
25 |
88.36 |
|
Day 2c
|
17 |
100 |
20 |
2 |
90.36 |
|
18 |
100 |
40 |
4 |
94.36 |
|
19 |
100 |
80 |
8 |
102.36 |
|
20 |
100 |
160 |
16 |
118.36 |
|
21 |
100 |
200 |
20 |
138.36 |
|
22 |
100 |
250 |
25 |
163.36 |
|
23 |
100 |
250 |
25 |
188.36 |

Five years later, in 2022, the COVID-19 pandemic became a global problem, and the patient revisited the allergy clinic seeking both COVID-19 vaccination and a follow-up colonoscopy. A vector-based vaccine that did not contain PEG could have been administered; however, PS80-containing vaccines were not easily available in Korea in early 2022, and the patient preferred mRNA vaccines. We decided to reattempt oral desensitization and, if successful, planned to perform a colonoscopy and administer the mRNA COVID-19 vaccine.
The patient was desensitized using the same protocol without premedication. After desensitization, the colonoscopy was safely performed. The day after the colonoscopy, she was successfully vaccinated with the first dose of the BNT162b2 vaccine. Three weeks later, desensitization to PEG was performed only on day 1 process, and the second vaccine was administered. The basophil HR test was performed, and HR was inhibited after desensitization, similar to the previous results (data not shown).
Ethics statement
Informed consent for desensitization as well as for publication of clinical data was obtained from the patient.
Go to :

DISCUSSION
Patients with immediate reactions to PEG require allergy workups, including skin test and/or basophil activation test
8; however, these approaches have not yet been standardized. A recent study recommended a titrated, stepwise approach for SPT with increasing concentrations of PEG up to PEG20000.
10 This approach was recommended due to skin reactivity to PEG potentially decreasing over time, resulting in false negative results.
10 Moreover,
in vitro HR test may have limitations, such as nonreleasing basophils. The patient in this report was negative in SPT but showed a positive reaction to PEG in IDT and significant HR to PEG. A positive IDT result and specific HR suggested possible involvement of IgE-mediated mechanisms in PEG hypersensitivity. After desensitization, basophil HR to PEG was significantly reduced. Thus, desensitization may suppress basophil and mast cell activity.
Two recent studies have extended our understanding of PEG allergy. Wenande et al.
1 described the clinical picture of 37 cases of immediate hypersensitivity to PEG published since 1977, proposing a diagnostic algorithm for PEG hypersensitivity. Stone et al.
2 identified two patients with hypersensitivity to PEG3350 and detected PEG-specific IgE and IgG antibodies. They also reported 53 additional cases of PEG3350 anaphylaxis and suggested that PEG-associated anaphylaxis may occur in approximately four cases per year.
2 The true prevalence of PEG allergy is unknown but is suspected to be underestimated due to the complex clinical features and difficulty in diagnosis. However, with increased attention to PEG, the incidence is expected to rise.
10 A recent study showed that 5–9% of 1,721 serum samples were positive for anti-PEG IgG, and two of 2,091 were positive for anti-PEG IgE, suggesting extensive exposure and sensitization to PEG in the general population.
11
PEG has been of great interest since mass vaccination against COVID-19 began. Two and six cases of anaphylaxis in the United Kingdom and United States, respectively, within the first days of initiating vaccination with the Pfizer-BioNTech vaccine led to suspicion of PEG being the culprit.
3 Incidences of anaphylaxis after COVID-19 vaccines have been reported worldwide, with varying rates according to region, population, and type of vaccines.
6 In the United States, a total of 66 cases of anaphylaxis were reported out of > 17 million doses of the mRNA COVID-19 vaccines administered between December 14, 2020 and January 18, 2021 (0.37 cases per 100,000 doses).
4 The World Allergy Organization estimated the incidence of anaphylaxis as about 1 in 200,000 doses for the Pfizer-BioNTech vaccine and 1 in 360,000 for the Moderna vaccine.
5 The Korea Disease Control and Prevention Agency (KDCA) recently reported adverse reactions in the COVID-19 vaccination registration system.
12 In Korea, a total of > 83 million doses of COVID-19 vaccines were administered from February 26 to November 20, 2021, and 380,715 cases (458.5 cases per 100,000 doses) of adverse reactions were reported. Of these, 1,509 patients had anaphylaxis-like reactions, and the estimated incidence rate of anaphylaxis was 1.8 cases per 100,000 doses. However, the actual rate may be much lower than this, as it is a crude calculation without causality assessments.
612 Nevertheless, anaphylaxis cases after COVID-19 vaccines have occurred, and many of them are likely caused by PEG.
Vaccines other than mRNA vaccines can safely be administered to patients with allergy to PEG.
81314 However, in early 2022, the use of vector-based vaccines dramatically decreased, making them difficult to obtain in Korea. An alternative approach may be to administer the COVID-19 mRNA vaccines through desensitization, and several related studies have been reported recently. AlMuhizi et al
15 reported successful desensitization by increasing the volume of undiluted vaccine to six patients with hypersensitivity reaction to their first dose of mRNA vaccine. It has also been reported that vaccination occurred safely after titrated challenge to six patients with positive skin tests to the vaccine, and desensitization using the BNT162b2 was effective in a patient sensitized to PS80.
1617 In all of these cases, desensitization was a graded challenge that started with 0.005–0.05 mL of undiluted vaccine and was increased in 4–5 steps. However, the safety and efficacy of graded challenges for COVID-19 vaccines have not been established. Failure of desensitization has been reported.
1819 It is also not clear whether this was a desensitization to PEG or to other components of the vaccine. In addition, the Korean government directly manages the distribution of COVID-19 vaccines; thus, it was impossible to directly administer the COVID-19 vaccine to the patient in our hospital. Furthermore, although the COVID-19 vaccine can be administered, there was a concern about the safe administration of laxatives for colonoscopy by graded challenges in our patient. Since no alternative vaccination method or alternative laxatives for colonoscopy was available, we made a shared decision with the patient to try oral, rather than injection, desensitization, and obtained good results.
In conclusion, oral desensitization to PEG in a patient with PEG allergy was successfully achieved. COVID-19 vaccination and bowel preparation are concerns in patients with PEG allergy. Oral desensitization to PEG may be an effective treatment strategy for patients with PEG allergy.
Go to :







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download