Non-tuberculous mycobacterial-pulmonary disease (NTM-PD) is a chronic infectious lung disease caused by various NTM species that are increasing rapidly worldwide.
12 The pathogenesis of NTM-PD has not been determined, but it might be related to predisposing alterations in host defense mechanisms, such as decreased protective ability of the respiratory cilia or suppressed cytokine response.
34 In addition, several chronic structural lung diseases including bronchiectasis, asthma, and chronic obstructive pulmonary disease (COPD) increase one’s risk of NTM-PD.
5 As a result, other respiratory infections, such as pulmonary tuberculosis and pulmonary aspergillosis, often co-exist in patients with NTM-PD.
67
Coronavirus disease 2019 (COVID-19) has threatened global health due to its persistent mutations.
89 The Centers for Disease Control and Prevention (CDC) recommends that patients with chronic respiratory diseases should be vaccinated against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Considering the mucociliary dysfunction, impaired host immunity, and frequent coexistence of other structural lung diseases in patients with NTM-PD,
10 the incidence of COVID-19 might be higher in these vulnerable patients than in the general population. However, there have been no studies on the incidence of COVID-19 in patients with NTM-PD. Therefore, our study aimed to investigate the incidence of and risk factors for COVID-19 in patients with NTM-PD, before the implementation of SARS-CoV-2 vaccination.
We retrospectively analyzed patient data obtained from January 1, 2010 to February 28, 2021. Data were extracted from the Clinical Data Warehouse Darwin-C of Samsung Medical Center (a 1,960-bed university-affiliated, tertiary referral hospital). We defined NTM-PD as the presence of both 10th International Classification of Disease Codes (ICD-10) of A31.0, A31.8, or A31.9 and microbiological findings (≥ 2 positive culture results from separate expectorated sputum, or ≥ 1 positive culture results from bronchial washing, bronchoalveolar lavage, or lung biopsy) from January 1, 2010 to December 31, 2019, before the onset of the COVID-19 pandemic. In the present study, NTM-PD was defined as a disease caused by one of the following three species: 1) mycobacterium avium complex (MAC;
M. avium and
M. intracellulare), 2)
M. abscessus complex (MABC;
M. abscessus subspecies abscessus and
M. abscessus subspecies massiliense), and 3)
M. kansasii. Those species were identified using a nested multiplex polymerase chain reaction and a reverse-hybridization assay of the internal transcribed spacer region of the
rpoB gene.
11 Finally, 3,866 patients aged ≥ 20 years with NTM-PD were included.
Treatment for NTM-PD was defined as concurrent use of the following drugs for more than 30 days: 1) a macrolide antibiotic and 2) one of the companion drugs (amikacin, clofazimine, ethambutol, rifamycin, linezolid, moxifloxacin, imipenem, cefoxitin, or tigecycline). NTM-PD patients were treated at the discretion of the attending physician.
12 Patients were classified into either the untreated group or the treated group according to whether they had received NTM treatment.
The outcome was incidence of COVID-19, which was defined as the presence of ICD-10 codes (U07, U08, or U09) from January 1, 2020 to February 28, 2021. During this period, COVID-19 vaccinations were not yet available.
Comorbidities were defined using ICD-10 codes, which were registered between the date of diagnosis of NTM-PD and December 31, 2019. Following diseases were included by referring to the previous study on the relationship between COVID-19 infection and chronic diseases: 1) hypertension (I10–I13 or I15) 2) diabetes mellitus (E11–E14) 3) COPD (J42–J44, except J43) 4) cancer (C00–C96).
13
The incidence of COVID-19 in the general population was calculated using data obtained from the Korea Disease Control and Prevention Agency. The incidence of COVID-19 was standardized for age using the 2005 Korean population. In the multivariable logistic regression analysis, the adjusted odds ratio (aOR) and 95% confidence interval (CI) for the treated group were calculated after adjusting for age, sex, NTM species, and comorbidities. NTM species were categorized as MAC, MABC, and others (M. kansasii or mixed infection). All statical analyses were performed using R 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria).
In patients with NTM-PD, the median (interquartile range) age was 62 (54–70) years, and the majority (62.0%) was female. Among 3,866 patients, 1,892 (48.9%) had initiated treatment for NTM-PD. The treated group was younger than the untreated group (received treatment vs. not received treatment: 61 [53–70] vs. 62 [55–71] years, P < 0.001). However, there was no difference in the distribution of men between the two groups (36.8% vs. 39.1%, P = 0.155). Significant intergroup differences (P < 0.001) were noted in the isolated NTM species as follows: MAC (78.4% vs. 72.7%), MABC (15.0% vs. 21.8%), and others (6.6% vs. 5.5%). Regarding comorbidities, the prevalence of COPD (40.8% vs. 24.0%, P < 0.001) was higher, but the prevalence of hypertension (12.3% vs. 14.9%, P = 0.022) and cancer (19.2% vs. 22.4%, P = 0.014) was lower in the treated group than in the untreated group. There was no significant difference in the prevalence of diabetes mellitus between the two groups (7.6% vs. 8.9%, P = 0.140).
From January 2020 to February 2021, 53 (1.4%) patients were infected with COVID-19. Meanwhile, 0.2% of the general population aged ≥ 20 years became infected with COVID-19 during the same period. In the age-stratified analysis, the age-standardized incidence of COVID-19 was higher in patients with NTM-PD than in the general population at all age groups (2.1% vs. 0.2%) (
Fig. 1). Regarding NTM treatment, 16 (0.8%) in the untreated group and 37 (2.0%) in the treated group were infected with COVID-19. In the treated group, the age-standardized incidence of COVID-19 was higher than in all patients with NTM-PD (3.4% vs. 2.1%). Among patients with NTM-PD, multivariable analysis showed that the patients who received NTM treatment were associated with an increased odd of COVID-19 compared to those who did not receive NTM treatment (aOR, 1.99; 95% CI, 1.09–3.64;
P = 0.026) (
Fig. 2).
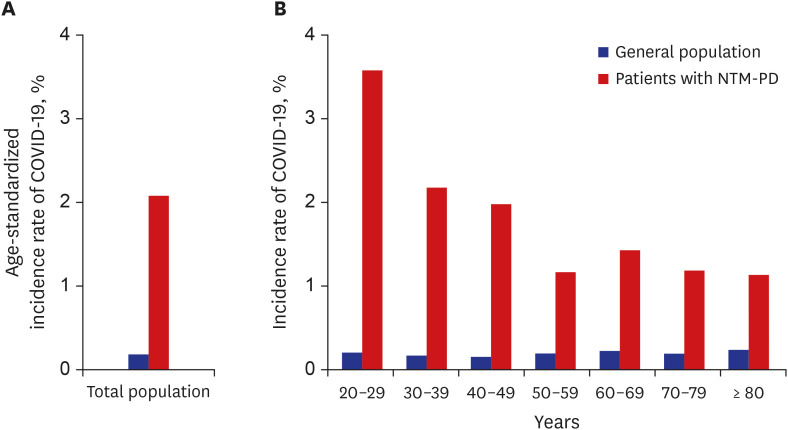 | Fig. 1
The incidence of coronavirus-2019 disease in the general population and in patients with non-tuberculous mycobacterial pulmonary disease. (A) age-standardized incidence of COVID-19. (B) incidence of COVID-19 stratified by age.
NTM-PD = non-tuberculous mycobacterial pulmonary disease, COVID-19 = coronavirus disease 2019.

|
 | Fig. 2
The odds for coronavirus-2019 disease in patients with non-tuberculous mycobacterial pulmonary disease according to treatment initiation for non-tuberculous mycobacterial pulmonary disease.
OR = odds ratio, CI = confidence interval, COVID-19 = coronavirus disease 2019, NTM = non-tuberculous mycobacteria.

|
Using the data collected before the vaccination against SARS-CoV-2 started, we found that the age-standardized incidence of COVID-19 is substantially higher in patients with NTM-PD than in the general population, particularly in patients who received the treatment for NTM-PD.
While the incidence of COVID-19 was relatively lower in Korea compared to many Western countries during the study period,
14 the incidence of COVID-19 in patients with NTM-PD was exceedingly higher than that of the general Korean population. Although the exact mechanism for this result has not been identified, there are some plausible explanations. Host factors that predisposed the patients to NTM-PD might have played a role as risk factors for increased susceptibility to COVID-19. Recent study using lung single cell RNA-sequencing data of patients with chronic lung disease showed that epithelial cells of these patients had pre-existing dysregulation of genes related to viral infection and innate immune response.
15 Impairment in airway-surface defense mechanisms that resulted from NTM-PD progression also can contribute to a further increased risk of contracting COVID-19. This relationship was supported by the higher risk of COVID-19 infection in patients who received treatment for NTM-PD compared to those without any treatment, since disease severity is the major determinant of treatment decisions in NTM-PD. In addition, given the studies on patients with other chronic lung diseases,
161718 those with NTM-PD are more likely to have a poor clinical outcome following COVID-19. While this should be assessed in future studies, we carefully suggest that patients with NTM-PD might be regarded as part of the high-risk group for COVID-19. More aggressive preventative measures, such as COVID-19 vaccine booster shots, may need to be implemented in this vulnerable population.
Our study has some limitations. First, because the diagnosis of COVID-19 was defined using the ICD-10 codes from a single institution, any diagnoses of COVID-19 made in other healthcare facilities were not captured. Therefore, the incidence of COVID-19 in patients with NTM-PD is likely underestimated in this study, and patients with NTM-PD might be at an even higher risk of COVID-19 compared to the general population. Second, the diagnoses of NTM-PD were defined by the presence of both ICD-10 codes and the microbiological findings in our study. Therefore, those who have mild or asymptomatic NTM-PD, or those with undiagnosed NTM-PD might have not been included in this study. Similarly, given that the study was conducted at the tertiary referral hospital, NTM-PD patients included in this study are likely to have more severe diseases than those with NTM-PD in general. This might have contributed to the higher incidence of COVID-19 in our study. Likewise, these patients with more severe NTM-PD were more likely to be tested for COVID-19 owing to their respiratory symptoms and more frequent medical encounters. However, the general population in Korea has very high accessibility to COVID-19 testing during the study period.
19 Third, detailed information regarding the severity of the NTM-PD, such as inflammatory markers, smear positivity, and cavities in chest images, were lacking. Instead, we estimated the disease severity based upon the presence of NTM treatment initiation because clinicians comprehensively evaluate the necessity for treatment based on disease severity. However, some patients with NTM-PD could not receive treatment due to side effects despite the severe disease. For instance, patients with poor health status or those who were intolerable to antibiotics treatment and discontinued before 30 days might have been classified into the untreated group. In this context, those who were treated for NTM-PD were younger and might have more active behavior, which increased their chance to be exposed to COVID-19. Finally, this study was conducted in Korea, where the incidence of COVID-19 was significantly lower than that in many Western countries during the study period, which limits the generalizability of these results to other populations. Nevertheless, the major strength of our study is the inclusion of a large number of patients with NTM-PD, who were defined by both ICD-10 codes and the microbiological findings. To the best of our knowledge, this is the first study to report the incidence of COVID-19 in patients with NTM-PD.
In conclusion, the incidence of COVID-19 might be higher in patients with NTM-PD than in the general population, particularly in those who required treatment for NTM-PD. An appropriate preventive strategy for COVID-19 or other pandemics in the future may be required in patients with NTM-PD.
Ethics statement
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board (IRB) of Samsung Medical Center (IRB No. SMC 2021-10-003). The need for written informed consent was waived because this was a retrospective analysis that used anonymized data.
Go to :







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download