INTRODUCTION
Fontan palliation is the current standard method of care for patients with a functionally univentricular hearts. Unfortunately, its life-threatening complications can include protein-losing enteropathy (PLE), liver cirrhosis, and plastic bronchitis. PLE is characterized by an abnormal loss of proteins, such as albumin, immunoglobulin, and clotting factor, into the gastrointestinal tract.
1) It has been reported to occur in 5–15% of patients after the Fontan operation and yield mortality rate of 50% at 5 years after the initial diagnosis in the 1990s.
2)3)4)
The precise pathophysiology of PLE after the Fontan operation is poorly understood; however, several factors contribute to diverse combinations of the individual cases. To the best of our knowledge, a higher systemic venous pressure and a low cardiac output states may be related to an altered mesenteric circulation that develops into PLE after the Fontan operation.
5) In response to a chronic low cardiac output, relatively inflamed state occurs, altering enterocyte membrane permeability and contributing to protein leakage.
5)6) Other factors, such as genetic disposition and predisposed variable lymphatic anatomy, are associated with developing PLE.
6)7)
Various treatments have been considered on the basis of various hypotheses regarding the pathophysiology of PLE, including medical, interventional, and surgical therapies.
8)9)10) However, only limited reports on the clinical characteristics, treatment responses, and outcomes of the patients who have developed Fontan-associated PLE are available. Considering the associated potentially life-threatening conditions, it is important to scrutinize which approaches fail or succeed in patients with PLE.
We performed a retrospective study of Fontan-associated PLE in the current era. This study aimed to investigate the survival rate and disease resolution in patients with PLE who underwent Fontan operation, as well as determine the predictive factors of mortality at the time of PLE diagnosis and review the treatment strategies used in patients with controlled PLE in 2 institutions in Korea.
RESULTS
Patient demographics
A total of 38 patients with Fontan-associated PLE were identified. PLE developed in 4.6% of the 832 patients after the Fontan operation, performed between 1986 and 2016 in the 2 institutions. The mean age at PLE diagnosis was 11.6±7.0 years, and PLE developed at a mean age of 7.7±6.1 years after the Fontan operation. The mean follow-up duration after PLE diagnosis was 8.9±5.9 years.
The dominant ventricular morphology was the right ventricle in 22 patients (58%). Heterotaxia syndrome was observed in 11 patients (29%). The most common clinical manifestation of PLE at the initial diagnosis was edema. Esophagogastroduodenoscopy was performed in 15 patients, and intestinal lymphangiectasia in the duodenum was documented in 10 patients.
At the time of PLE diagnosis, there were 29 and 9 patients with New York Heart Association (NYHA) functional classification I or II and III or IV, respectively. RLC was found in 23 patients, and anemia in 16 patients. Fifteen patients had a history of tachyarrhythmia, including atrial flutter and atrial fibrillation. Six patients underwent pacemaker insertion for sinus node dysfunction and complete atrioventricular block.
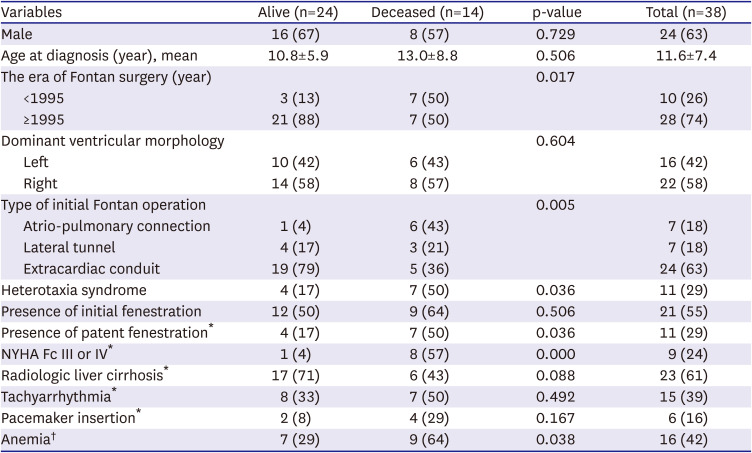
Table 1 shows the clinical characteristics of the study patients at the time of PLE diagnosis.
Table 1
Clinical characteristics at the time of PLE diagnosis in the alive and deceased patient groups
|
Variables |
Alive (n=24) |
Deceased (n=14) |
p-value |
Total (n=38) |
|
Male |
16 (67) |
8 (57) |
0.729 |
24 (63) |
|
Age at diagnosis (year), mean |
10.8±5.9 |
13.0±8.8 |
0.506 |
11.6±7.4 |
|
The era of Fontan surgery (year) |
|
|
0.017 |
|
|
<1995 |
3 (13) |
7 (50) |
10 (26) |
|
≥1995 |
21 (88) |
7 (50) |
28 (74) |
|
Dominant ventricular morphology |
|
|
0.604 |
|
|
Left |
10 (42) |
6 (43) |
16 (42) |
|
Right |
14 (58) |
8 (57) |
22 (58) |
|
Type of initial Fontan operation |
|
|
0.005 |
|
|
Atrio-pulmonary connection |
1 (4) |
6 (43) |
7 (18) |
|
Lateral tunnel |
4 (17) |
3 (21) |
7 (18) |
|
Extracardiac conduit |
19 (79) |
5 (36) |
24 (63) |
|
Heterotaxia syndrome |
4 (17) |
7 (50) |
0.036 |
11 (29) |
|
Presence of initial fenestration |
12 (50) |
9 (64) |
0.506 |
21 (55) |
|
Presence of patent fenestration*
|
4 (17) |
7 (50) |
0.036 |
11 (29) |
|
NYHA Fc III or IV*
|
1 (4) |
8 (57) |
0.000 |
9 (24) |
|
Radiologic liver cirrhosis*
|
17 (71) |
6 (43) |
0.088 |
23 (61) |
|
Tachyarrhythmia*
|
8 (33) |
7 (50) |
0.492 |
15 (39) |
|
Pacemaker insertion*
|
2 (8) |
4 (29) |
0.167 |
6 (16) |
|
Anemia†
|
7 (29) |
9 (64) |
0.038 |
16 (42) |
The median age at Fontan operation was 3.1 (IQR=2.5–4.6) years. The most common type of Fontan operation was extracardiac conduit (ECC) Fontan (n=24, 63%), followed by atriopulmonary (AP) Fontan (n=7, 18%), and lateral tunnel (LT) Fontan (n=7, 18%). PLE was developed in 4.9% (n=24/492), 6.1% (n=7/114), and 3.1% (n=7/226) of the patients who underwent ECC, AP, and LT Fontan operations, respectively.
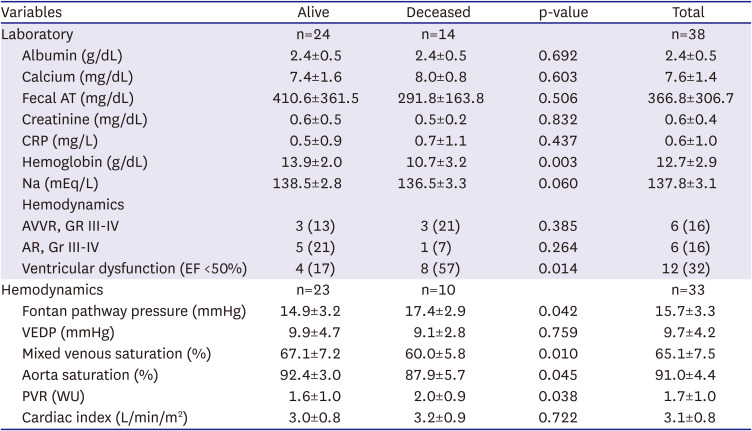
Twenty-one patients (55%) had initial Fontan pathway fenestration, among whom 11 patients had patent Fontan pathway fenestration at the initial PLE diagnosis. The duration from Fontan operation to the onset of PLE was 6.15±4.97 years in the initial fenestration group and 9.64±6.89 years in the non-fenestration group. However, there were no significant differences between the 2 groups. Fifteen patients (39%) underwent further heart surgery after the initial Fontan operation before PLE diagnosis; 2 patients, Fontan conversion; and 7 patients, valve surgery. The laboratory and hemodynamic data are summarized in
Table 2. The mean total serum albumin level was 2.4±0.5 g/dL at the initial PLE diagnosis. Twelve patients (32%) had ventricular dysfunction during PLE diagnosis.
Table 2
Laboratory and hemodynamic data at the time of protein-losing enteropathy diagnosis in alive and deceased patient groups
|
Variables |
Alive |
Deceased |
p-value |
Total |
|
Laboratory |
n=24 |
n=14 |
|
n=38 |
|
Albumin (g/dL) |
2.4±0.5 |
2.4±0.5 |
0.692 |
2.4±0.5 |
|
Calcium (mg/dL) |
7.4±1.6 |
8.0±0.8 |
0.603 |
7.6±1.4 |
|
Fecal AT (mg/dL) |
410.6±361.5 |
291.8±163.8 |
0.506 |
366.8±306.7 |
|
Creatinine (mg/dL) |
0.6±0.5 |
0.5±0.2 |
0.832 |
0.6±0.4 |
|
CRP (mg/L) |
0.5±0.9 |
0.7±1.1 |
0.437 |
0.6±1.0 |
|
Hemoglobin (g/dL) |
13.9±2.0 |
10.7±3.2 |
0.003 |
12.7±2.9 |
|
Na (mEq/L) |
138.5±2.8 |
136.5±3.3 |
0.060 |
137.8±3.1 |
|
Hemodynamics |
|
|
|
|
|
AVVR, GR III-IV |
3 (13) |
3 (21) |
0.385 |
6 (16) |
|
AR, Gr III-IV |
5 (21) |
1 (7) |
0.264 |
6 (16) |
|
Ventricular dysfunction (EF <50%) |
4 (17) |
8 (57) |
0.014 |
12 (32) |
|
Hemodynamics |
n=23 |
n=10 |
|
n=33 |
|
Fontan pathway pressure (mmHg) |
14.9±3.2 |
17.4±2.9 |
0.042 |
15.7±3.3 |
|
VEDP (mmHg) |
9.9±4.7 |
9.1±2.8 |
0.759 |
9.7±4.2 |
|
Mixed venous saturation (%) |
67.1±7.2 |
60.0±5.8 |
0.010 |
65.1±7.5 |
|
Aorta saturation (%) |
92.4±3.0 |
87.9±5.7 |
0.045 |
91.0±4.4 |
|
PVR (WU) |
1.6±1.0 |
2.0±0.9 |
0.038 |
1.7±1.0 |
|
Cardiac index (L/min/m2) |
3.0±0.8 |
3.2±0.9 |
0.722 |
3.1±0.8 |
Patients’ survival and predictive factors of mortality
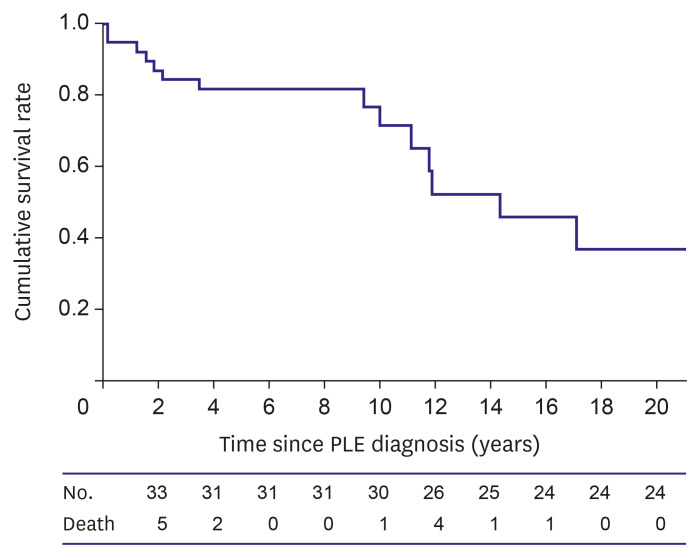
The Kaplan–Meier survival curve demonstrated that the survival rate was 81.6% at 5 years and 76.5% at 10 years after PLE diagnosis (
Figure 1). There were 14 mortalities, and the mean age at mortality was 19.9±10.9 years. The mean time from PLE diagnosis to mortality was 6.9±5.9 years. Five patients died of sepsis; four, respiratory complications; three, heart failure; and two, brain infarction or hemorrhage. Mortality in one patient was associated with reoperation of the Fontan takedown.
Figure 1
Overall survival curve in patients with PLE after the Fontan operation.
PLE = protein losing enteropathy.
The clinical characteristics and laboratory and hemodynamic data at the time of PLE diagnosis were compared between the alive and deceased patient groups (
Tables 1 and
2). The deceased patient group showed a poor NYHA functional classification and had a significantly higher incidence of ventricular dysfunction than the alive patient group. Heterotaxia syndrome and patent fenestration at the time of PLE diagnosis were more common in the deceased patient group than in the alive patient group.
Laboratory assessment revealed that the hemoglobin levels were significantly lower in the deceased patient group than in the alive patient group. (10.7±3.2 vs. 13.9±2.0, p=0.003). The hemodynamic assessment revealed that the deceased patient group showed a lower aortic oxygen saturation at the time of PLE diagnosis than did the alive patient group (87.9±5.7 vs. 92.4±3.0, p=0.045). In addition, the mixed venous saturation was lower in the deceased patient group than in the alive patient group. (60.0±5.8 vs. 67.1±7.2, p=0.010). The Fontan pathway pressure was higher in the deceased patient group than in the alive patient group (17.4± 2.9 vs. 14.9± 3.2, p=0.041). The severity of atrioventricular valve regurgitation, initial serum albumin level, ventricular end-diastolic pressure, cardiac index, pulmonary vascular resistance index, concomitant liver cirrhosis, and tachyarrhythmia were not associated with mortality.
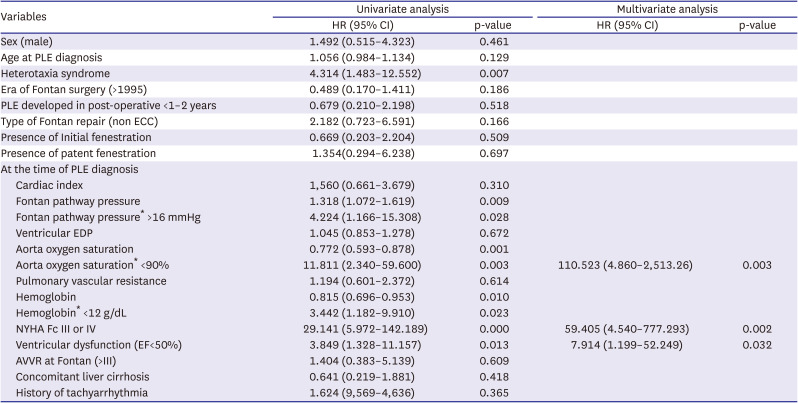
Table 3 presents the results of the Cox proportional hazard regression analysis for the potential predictive factors of mortality in the patients with Fontan-associated PLE. Heterotaxia syndrome (HR=4.31, p=0.007), NYHA functional classification III or IV (HR=29.14, p<0.0001), higher Fontan pathway pressure (>16 mmHg) (HR=4.22, p=0.028), and ventricular dysfunction (HR=3.85, p=0.013), as well as a low aortic oxygen saturation (<90%) (HR=11.81, p=0.003) and low hemoglobin level (<12 g/dL) (HR=3.44, p=0.023) at the time of PLE diagnosis, were found as the predictive factors of mortality. In the multivariate Cox proportional hazards regression analysis, NYHA functional classification III or IV (HR=59.41, p=0.002), low aortic oxygen saturation (<90%) (HR=110.52, p=0.003), ventricular dysfunction (EF of <50%) (HR=7.91, p=0.032) were independently associated with mortality.
Table 3
Cox proportional hazard regression analysis for predictive factor of mortality in Fontan patients with PLE
|
Variables |
Univariate analysis |
Multivariate analysis |
|
HR (95% CI) |
p-value |
HR (95% CI) |
p-value |
|
Sex (male) |
1.492 (0.515–4.323) |
0.461 |
|
|
|
Age at PLE diagnosis |
1.056 (0.984–1.134) |
0.129 |
|
|
|
Heterotaxia syndrome |
4.314 (1.483–12.552) |
0.007 |
|
|
|
Era of Fontan surgery (>1995) |
0.489 (0.170–1.411) |
0.186 |
|
|
|
PLE developed in post-operative <1–2 years |
0.679 (0.210–2.198) |
0.518 |
|
|
|
Type of Fontan repair (non ECC) |
2.182 (0.723–6.591) |
0.166 |
|
|
|
Presence of Initial fenestration |
0.669 (0.203–2.204) |
0.509 |
|
|
|
Presence of patent fenestration |
1.354(0.294–6.238) |
0.697 |
|
|
|
At the time of PLE diagnosis |
|
|
|
|
|
Cardiac index |
1,560 (0.661–3.679) |
0.310 |
|
|
|
Fontan pathway pressure |
1.318 (1.072–1.619) |
0.009 |
|
|
|
Fontan pathway pressure* >16 mmHg |
4.224 (1.166–15.308) |
0.028 |
|
|
|
Ventricular EDP |
1.045 (0.853–1.278) |
0.672 |
|
|
|
Aorta oxygen saturation |
0.772 (0.593–0.878) |
0.001 |
|
|
|
Aorta oxygen saturation* <90% |
11.811 (2.340–59.600) |
0.003 |
110.523 (4.860–2,513.26) |
0.003 |
|
Pulmonary vascular resistance |
1.194 (0.601–2.372) |
0.614 |
|
|
|
Hemoglobin |
0.815 (0.696–0.953) |
0.010 |
|
|
|
Hemoglobin* <12 g/dL |
3.442 (1.182–9.910) |
0.023 |
|
|
|
NYHA Fc III or IV |
29.141 (5.972–142.189) |
0.000 |
59.405 (4.540–777.293) |
0.002 |
|
Ventricular dysfunction (EF<50%) |
3.849 (1.328–11.157) |
0.013 |
7.914 (1.199–52.249) |
0.032 |
|
AVVR at Fontan (>III) |
1.404 (0.383–5.139) |
0.609 |
|
|
|
Concomitant liver cirrhosis |
0.641 (0.219–1.881) |
0.418 |
|
|
|
History of tachyarrhythmia |
1.624 (9,569–4,636) |
0.365 |
|
|
Treatment and response
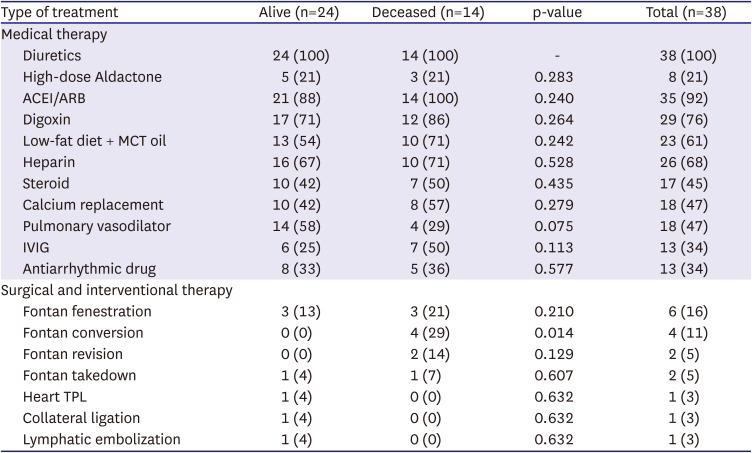
Twenty-one patients (55%) received medical therapy only, while 17 patients (45%) received medical plus surgical or interventional therapy. The types of therapy used are listed in
Table 4. All patients received supportive medical therapy, such as diuretics administration and intravenous albumin replacement. Subcutaneous unfractionated heparin was used in 26 patients (68%), steroids in 17 patients (45%), and pulmonary vasodilators, such as sildenafil and beraprost, in 18 patients (47%).
Table 4
Type of treatment in alive and deceased patient group
|
Type of treatment |
Alive (n=24) |
Deceased (n=14) |
p-value |
Total (n=38) |
|
Medical therapy |
|
|
|
|
|
Diuretics |
24 (100) |
14 (100) |
- |
38 (100) |
|
High-dose Aldactone |
5 (21) |
3 (21) |
0.283 |
8 (21) |
|
ACEI/ARB |
21 (88) |
14 (100) |
0.240 |
35 (92) |
|
Digoxin |
17 (71) |
12 (86) |
0.264 |
29 (76) |
|
Low-fat diet + MCT oil |
13 (54) |
10 (71) |
0.242 |
23 (61) |
|
Heparin |
16 (67) |
10 (71) |
0.528 |
26 (68) |
|
Steroid |
10 (42) |
7 (50) |
0.435 |
17 (45) |
|
Calcium replacement |
10 (42) |
8 (57) |
0.279 |
18 (47) |
|
Pulmonary vasodilator |
14 (58) |
4 (29) |
0.075 |
18 (47) |
|
IVIG |
6 (25) |
7 (50) |
0.113 |
13 (34) |
|
Antiarrhythmic drug |
8 (33) |
5 (36) |
0.577 |
13 (34) |
|
Surgical and interventional therapy |
|
|
|
|
|
Fontan fenestration |
3 (13) |
3 (21) |
0.210 |
6 (16) |
|
Fontan conversion |
0 (0) |
4 (29) |
0.014 |
4 (11) |
|
Fontan revision |
0 (0) |
2 (14) |
0.129 |
2 (5) |
|
Fontan takedown |
1 (4) |
1 (7) |
0.607 |
2 (5) |
|
Heart TPL |
1 (4) |
0 (0) |
0.632 |
1 (3) |
|
Collateral ligation |
1 (4) |
0 (0) |
0.632 |
1 (3) |
|
Lymphatic embolization |
1 (4) |
0 (0) |
0.632 |
1 (3) |
Some patients with specific hemodynamic abnormalities at the time of PLE diagnosis also received surgical or interventional therapies. Fontan fenestration and conversion were performed in 6 and 4 patients, respectively. Fontan conversion from APC to ECC after PLE diagnosis was associated with mortality during follow-up (p=0.014). Most patients who underwent fenestration had a high Fontan pathway pressure of >16 mmHg. Two patients underwent Fontan pathway revision for Fontan pathway stenosis and Fontan take-down. Heart transplantation was performed in one patient with PLE accompanied with ventricular failure.
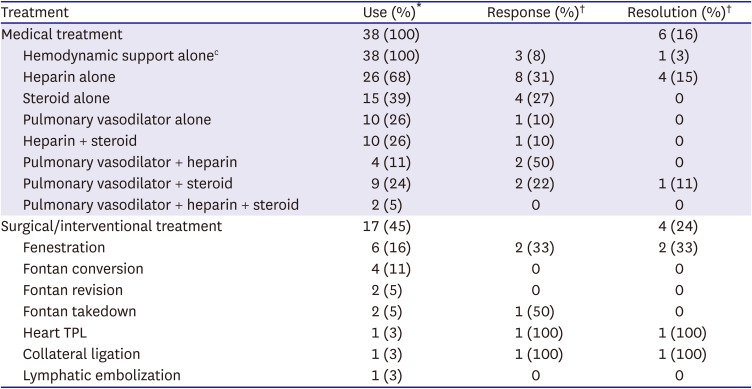
Table 5 shows the response and resolution rates for each treatment in the patients with Fontan-associated PLE. There was no significant difference in the clinical characteristics and laboratory and hemodynamic data between the resolution and non-resolution groups at the time of PLE diagnosis. In the resolution group, 6 patients experienced PLE resolution with medical therapy alone. One patient showed resolution with hemodynamically supportive medication alone. Heparin was frequently used in our study patients. The rate of initial response to heparin was 30%. However, only 15% of the patients showed resolution while maintaining heparin injection. These patients showed features of heparin dependency during the course of the treatment. However, this rate was a relatively high, considering that the other treatments were not remarkably effective. An initial heparin response was associated with survival in our study patients (p=0.043). Heparin administration resulted in resolution in 4 patients.
Table 5
Response and resolution rate of each treatment for patients with protein losing enteropathy
|
Treatment |
Use (%)*
|
Response (%)†
|
Resolution (%)†
|
|
Medical treatment |
38 (100) |
|
6 (16) |
|
Hemodynamic support alonec
|
38 (100) |
3 (8) |
1 (3) |
|
Heparin alone |
26 (68) |
8 (31) |
4 (15) |
|
Steroid alone |
15 (39) |
4 (27) |
0 |
|
Pulmonary vasodilator alone |
10 (26) |
1 (10) |
0 |
|
Heparin + steroid |
10 (26) |
1 (10) |
0 |
|
Pulmonary vasodilator + heparin |
4 (11) |
2 (50) |
0 |
|
Pulmonary vasodilator + steroid |
9 (24) |
2 (22) |
1 (11) |
|
Pulmonary vasodilator + heparin + steroid |
2 (5) |
0 |
0 |
|
Surgical/interventional treatment |
17 (45) |
|
4 (24) |
|
Fenestration |
6 (16) |
2 (33) |
2 (33) |
|
Fontan conversion |
4 (11) |
0 |
0 |
|
Fontan revision |
2 (5) |
0 |
0 |
|
Fontan takedown |
2 (5) |
1 (50) |
0 |
|
Heart TPL |
1 (3) |
1 (100) |
1 (100) |
|
Collateral ligation |
1 (3) |
1 (100) |
1 (100) |
|
Lymphatic embolization |
1 (3) |
0 |
0 |
The rate of initial response to steroids was similar to that to heparin. However, the albumin levels fluctuated depending on the steroid dose, which ultimately resulted in failed therapy. Steroid administration with adjuvant pulmonary vasodilator medication yielded resolution in one patient even though the albumin levels changed with the steroid dose. Herein, we found no evidence on the effectiveness of steroid or pulmonary vasodilator therapy alone in our study.
Selectively applied surgical or interventional therapies yielded some resolution. Two of 6 patients who underwent Fontan fenestration creation showed resolution of PLE. Fontan conversion to ECC was performed to treat Fontan failure in 4 patients. However, Fontan conversion was not adequate for treating PLE in all patients, and all patients died during follow-up. Two patients who underwent Fontan revision for Fontan pathway obstruction also did not respond to the treatment. Recently, one patient underwent delayed Fontan takedown and conversion to pulsatile bidirectional cavopulmonary shunt (BCPS) to treat PLE and severe hepatic dysfunction.
11) This patient had an improved PLE with adjuvant steroid and pulmonary vasodilator therapy after surgery during a 2.1-year follow-up period. The patients who underwent aortopulmonary collateral ligation and heart transplantation showed PLE resolution. Recently, intestinal lymphatic embolization was attempted in one patient; unfortunately, there was no effect observed during the 1-year follow-up.
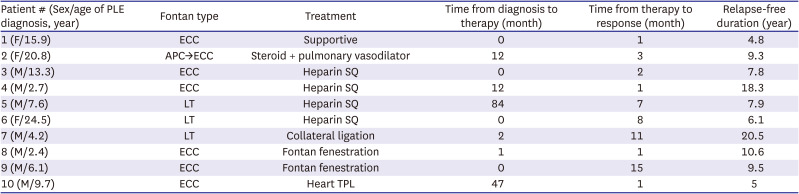
Table 6 summarizes the 10 patients who achieved PLE resolution. The definitive modality was applied median 1.5 months [IQR 0.0–12.0] after the PLE diagnosis for resolution in these patients. They showed a response after median 2.5 months [IQR 1.0–8.0] of applying an effective treatment, and finally, they remained in a resolution state for median 8.6 years [IQR 6.1–10.6] with or without medication. However, 53% (20/38) of the total patients with PLE showed no improvement despite treatment, and half of them finally died. Intractable PLE was significantly associated with late mortality in the patients with Fontan-associated PLE (p=0.015).
Table 6
Summary of patients with protein losing enteropathy resolution
|
Patient # (Sex/age of PLE diagnosis, year) |
Fontan type |
Treatment |
Time from diagnosis to therapy (month) |
Time from therapy to response (month) |
Relapse-free duration (year) |
|
1 (F/15.9) |
ECC |
Supportive |
0 |
1 |
4.8 |
|
2 (F/20.8) |
APC→ECC |
Steroid + pulmonary vasodilator |
12 |
3 |
9.3 |
|
3 (M/13.3) |
ECC |
Heparin SQ |
0 |
2 |
7.8 |
|
4 (M/2.7) |
ECC |
Heparin SQ |
12 |
1 |
18.3 |
|
5 (M/7.6) |
LT |
Heparin SQ |
84 |
7 |
7.9 |
|
6 (F/24.5) |
LT |
Heparin SQ |
0 |
8 |
6.1 |
|
7 (M/4.2) |
LT |
Collateral ligation |
2 |
11 |
20.5 |
|
8 (M/2.4) |
ECC |
Fontan fenestration |
1 |
1 |
10.6 |
|
9 (M/6.1) |
ECC |
Fontan fenestration |
0 |
15 |
9.5 |
|
10 (M/9.7) |
ECC |
Heart TPL |
47 |
1 |
5 |
DISCUSSION
Herein, we present a long-term follow-up of 11.6 years of study of Fontan-associated PLE in 2 large institutions in Korea. To the best of our knowledge, this is the first study to describe the outcomes of Fontan-associated PLE in the country. This study aimed to investigate the clinical and hemodynamic characteristics of patients with Fontan-associated PLE and evaluate the treatment strategies. Furthermore, we evaluated the survival rate and factors related to mortality among these patients.
Historically, the survival rate of patients with PLE was approximately 60% at 5 years after diagnosis in the 1990s.
4) However, in our study, the survival rate of the patients with PLE was 81.6% at 5 years and 76.5% at 10 years. More contemporary studies have shown a better survival rate of up to 88% at 5 years after diagnosis of PLE.
9)12) With the evolution of surgical techniques for the Fontan operation, there has been an improvement in the overall survival rate; however, PLE remains as high-risk factor for mortality after the Fontan operation.
13) Earlier diagnosis of PLE, comprehensive assessment of the causes of the PLE, and versatile aggressive treatment can increase patient survival. Nevertheless, there is still an irrefutable mortality rate in patients with PLE, and it is challenging to control PLE.
12)14)
In our study, we identified the factors associated with mortality based on the clinical characteristics and laboratory and hemodynamic data at the time of the initial PLE diagnosis. A high NYHA functional classification (grade III or IV), lower aortic oxygen saturation (<90%) at rest, and decreased ventricular function at the time of PLE diagnosis were associated with significant mortality in the patients with PLE after the Fontan operation. A low aortic oxygen saturation at rest was related to the presence of a fenestration or the development of venovenous collaterals with right-to-left shunting. Cyanosis can be considered a valuable surrogate parameter for impaired Fontan hemodynamics and impending Fontan failure.
15) Systemic ventricular dysfunction is the cause of decreased cardiac output and Fontan failure. Moreover, a high NYHA functional classification reflects reduced ventricular performance, which is well known to be associated with mortality in patients with heart failure. These factors are related to a low cardiac output and hemodynamic instability, and by themselves, affect mortality in patients undergoing Fontan operation. Unexpectedly, a low cardiac index was not a predictive factor of mortality in the patients with PLE in our study, despite it being the hallmark sign of failed Fontan circulation. This might be explained by compensatory mechanisms that maintain the cardiac output at the cost of central venous pressure or oxygen saturation.
16) Therefore, these factors might significantly impact deterioration in patients with PLE. Therefore, we believe that patients with PLE who display these high-risk characteristics associated with mortality should be evaluated immediately and managed more aggressively with multiple treatment modalities.
9)
Various strategies have been used to treat PLE; however, there is no single best strategy yet. As the cause of PLE is multifactorial and unclear, treatment strategies must be individualized. In fact, our long-term result on PLE treatment were not satisfactory. However, the survival rates have improved significantly, and the fact were some treatments inducing response is encouraging. In this sense, it is meaningful to analyze the effects of previous treatments on PLE.
It is crucial to comprehensively evaluate patients diagnosed with PLE using diverse tools. Any factors resulting in increased central venous pressure or decreased cardiac output will predispose Fontan patients with PLE. Atrioventricular valve regurgitation, residual outflow obstructive lesion, multiple collaterals, and tach-bradyarrhythmia, which result in atrioventricular dyssynchrony, should be considered for immediate correction. However, it must be recognized that there is a considerable disparity between hemodynamic deterioration findings and the manifestation of PLE in Fontan patients.
10) We already realize that some patients with PLE have suitable hemodynamic parameters. Even though poor hemodynamics, which are thought to be related to PLE, were corrected, PLE might not have improved. However, considering the high mortality rate of PLE, the abnormal laboratory and hemodynamic findings should be aggressively addressed as soon as possible. Moreover, extracardiac lesions and conditions that may worsen hemodynamics, including anemia and thyroid dysfunction, should be evaluated, and corrected immediately.
9)17)
Surgical and interventional therapies are commonly used in patients with hemodynamic problems. These therapies resulted in a dramatic resolution of PLE in selected patients in our study. Fontan fenestration can theoretically improve the cardiac output in patients with elevated central venous pressure and decompress the Fontan pathway pressure to relieve PLE, although at the expense of hypoxemia and increased stroke risk.
18)19) In our study, only one-third of the patients showed resolution of PLE after fenestration creation. Interestingly, responsiveness was not correlated with the Fontan pathway pressure, and there was no recurrence of PLE after spontaneous fenestration closure in either patient.
19) Fontan revision for primary PLE treatment did not yield good results in our study. All patients underwent Fontan conversion for various reasons, including PLE, however, all were ineffective as PLE treatment, and all patients eventually died. Fontan conversion to an ECC is an option for treating failing Fontan patients with improved cardiac output. AP Fontan operation often yields marked enlargement of the right atrium and less efficient flow dynamics.
20)21) However, PLE seems to derive limited benefit from Fontan conversion, and the reported mortality rate approaches 50%.
20) Notably, one patient with liver dysfunction and PLE underwent delayed Fontan takedown and pulsatile BCPS in our study. The albumin levels improved with adjuvant steroid pulse therapy and pulmonary vasodilator medication.
11)
Despite some promising cases, the effectiveness and proper timing of Fontan takedown are still unclear. It must be interpreted with caution that surgical or interventional procedures alone did not result in the resolution of PLE. Some surgical or interventional procedures can be effective when combine with other medical therapies.
Other methods that can improve the cardiac output, including aortopulmonary collateral vessel ligation
22) and correction of atrioventricular valve regurgitation, can be used in patients with PLE but have a limited effect on selected patients. Interventional thoracic duct decompression by rerouting the innominate vein to the atrium has been introduced.
23) Further, intestinal lymphatic embolization has been attempted for PLE treatment.
24) However, further studies are needed to determine the long-term outcomes of these procedures.
Heart transplantation has been reported to be a potential treatment strategy for Fontan failure. After transplantation, patients experience an immediate response; sometimes, PLE can take time to improve, and the patient will need additional treatment, such as a short course of steroids administration.
8) In a recent report, the risk of post-transplantation mortality in patients with PLE was acceptable, and PLE resolved in nearly all survivors.
25) As the survival rate of transplantation in Fontan patients improved, transplantation may also be considered a treatment option for PLE.
26) Selected high-risk patients with PLE may be considered for transplantation early if their clinical situation does not improve.
In our experience, some patients achieve resolution with medical therapy. Intestinal-directed therapy, including subcutaneous unfractionated heparin or corticosteroids, targeted to reduce enteric protein loss, is considered in most patients. Subcutaneous unfractionated heparin acts as a mechanical barrier by decreasing the permeability of the basal membrane to large molecules, such as albumin.
6) In our study, nearly one-third of the patients who received heparin therapy initially, whether combined with other therapies, showed a response. Finally, similar to another study,
27) our study found that 15% of the patients administered with heparin experienced resolution after continuing heparin use. Interestingly, our data suggest that good initial responsiveness to heparin might be associated with reduced mortality. However, since we did not equally apply heparins in all patients with PLE, and our analysis was unadjusted for other possible confounders, further studies are needed for verification.
Corticosteroids have anti-inflammatory effects, stabilize the intestinal capillary or lymphatic membrane, and decrease intestinal lacteal dilatation, membrane permeability, and infiltration of leukocytes.
6)28) In our study, 27% of the patients on oral steroid therapy showed a response. However, only one patient who underwent steroid and pulmonary vasodilator combination therapy had a resolution, although the albumin level waxed and waned depending on the steroid dose. Steroids also yielded an initial response similar to that of heparin, but which could not be sustained in our study. Furthermore, the complications of systemic steroid use, such as infection, growth failure, and gastric ulcers, are considerable problems in maintaining steroid use.
28)29) Patients with PLE are already immunocompromised, and long-term use of steroids makes them more susceptible to infection. Heparin or steroid therapy usually should be continued to maintain response. Heparin therapy may be associated with osteopenia or bleeding tendencies. However, complications occurred more frequently in the steroid use group than in the heparin use group. Combination therapy with a pulmonary vasodilator and heparin or a pulmonary vasodilator and steroids improved PLE in some patients. Pulmonary vasodilators can increase the mesenteric arterial flow by dilating the mesenteric vessels, increasing the cardiac output, and lowering the central venous pressure by reducing pulmonary vascular resistance.
30)
However, unfortunately, 53% of the total patients in our study had intractable PLE despite any treatment. Moreover, the present resolution of PLE is not permanent, indicating no “cure” for PLE. Maintaining the resolution state is essential but challenging. Nevertheless, it is encouraging that the 5-year survival after PLE diagnosis improved remarkably, and some patients maintained the resolution of PLE. These promising results may be attributed to the more aggressive and multimodal therapeutic approach individually used (
Figure 2).
Figure 2
Comprehensive evaluation and multidisciplinary treatment for Fontan-associated PLE.
AV = atrioventricular; CT = computed tomography; MRI = magnetic resonance imaging; PLE = protein losing enteropathy.

This study had several limitations. First, the sample size may have been too small to detect the influence of the variables. Moreover, the variations in the treatment concepts during long periods made statistical analyses difficult. Second, the information provided in this retrospective study is not universally available. Because this study had incomplete data, it was difficult to draw conclusions. Cardiac catheterization was not performed in all patients with PLE at the initial diagnosis. Third, center- and physician-related differences in the evaluation and first treatment choice could have been present in our study. Moreover, the hemodynamic criteria, which are thought to require intervention, were applied differently depending on the time and risk factors. Additionally, the combinations of therapeutic modalities may deter the unambiguous identification of individual treatment effects. Furthermore, to some extent, therapy effectiveness might have resulted in underestimation that partial responsiveness (if it is not fulfilled to the criteria of positive response or resolution) was challenging to evaluate. Therefore, these results should be carefully interpreted.
In conclusion, although survival has improved with the advancement of various treatments, PLE after the Fontan operation remains challenging to treat. The presence of high-risk characteristics associated with mortality should prompt evaluation and more aggressive and tailored management. Data on why this ailment occurs are limited, and there is no effective clear-cut treatment. However, through perseverant investigations and attempts to develop novel treatments, some studies have reported the resolution of PLE. Further investigations are needed to determine the exact mechanism of Fontan-associated PLE and to share new therapeutic approaches for improving patient outcomes.












 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download