Author's summary
By actively implementing contemporary management strategies in hypertrophic cardiomyopathy, morbidity and mortality can be substantially reduced. In this review, we discuss the pathophysiology and management of the major clinical issues in hypertrophic cardiomyopathy, including sudden cardiac death, atrial fibrillation and thromboembolism, dynamic left ventricular outflow tract obstruction, and heart failure progression. Although echocardiography and cardiac magnetic resonance imaging currently play an essential and complementary role in the management of hypertrophic cardiomyopathy, further studies are needed to establish how developing techniques such as myocardial deformation and late gadolinium enhancement can provide better risk stratification and guide treatment.
Abstract
Hypertrophic cardiomyopathy (HCM) is one of the most common inheritable cardiomyopathies. Contemporary management strategies, including the advent of implantable cardioverter-defibrillators and effective anticoagulation, have substantially improved the clinical course of HCM patients; however, the disease burden of HCM is still high in Korea. Sudden cardiac death (SCD), atrial fibrillation and thromboembolic risk, dynamic left ventricular outflow tract (LVOT) obstruction, and heart failure (HF) progression remain important issues in HCM. SCD in HCM can be effectively prevented with implantable cardioverter-defibrillators. However, appropriate patient selection is important for primary prevention, and the 5-year SCD risk score and the presence of major SCD risk factors should be considered. Anticoagulation should be initiated in all HCM patients with atrial fibrillation regardless of the CHA2DS2-VASc score, and non-vitamin K antagonist oral anticoagulants are the first option. Symptomatic dynamic LVOT obstruction is first treated medically with negative inotropes, and if symptoms persist, septal reduction therapy is considered. The recently approved myosin inhibitor mavacamten is promising. HF in HCM is usually related to diastolic dysfunction, while about 5% of HCM patients show reduced left ventricular ejection fraction <50%, also referred to as “end-stage” HCM. Myocardial fibrosis plays an important role in the progression to advanced HF in patients with HCM. Patients who do not respond to guideline-directed medical therapy can be considered for heart transplantation. The development of imaging techniques, such as myocardial deformation on echocardiography and late gadolinium enhancement on cardiac magnetic resonance, can provide better risk evaluation and decision-making for management strategies in HCM.
Graphical Abstract
Hypertrophic cardiomyopathy (HCM) as a disease entity was first introduced in the late 1950s in patients with significant subaortic pressure gradients and discrete hypertrophy of the interventricular septum, but without aortic stenosis, leading to coining of the term “idiopathic hypertrophic subaortic stenosis.”1)2) Since then, HCM has been noted to manifest with various patterns of left ventricular (LV) hypertrophy, with and without dynamic left ventricular outflow tract (LVOT) obstruction.3) Genetically, HCM is mainly caused by gene mutations of the cardiac sarcomeric complex with an autosomal dominant inheritance pattern, though the pathogenic gene variant is currently identifiable in only around 50% of the patients.4)5) HCM has been reported worldwide with a prevalence of 1:200 to 1:500; however, many individuals affected with HCM are under-recognized, especially in minorities and developing countries, and the currently diagnosed population of HCM represent only the tip of the iceberg.6)7) The prevalence and incidence of HCM are gradually increasing in Korea as well as worldwide, which is likely related to heightened clinical awareness of the disease;8) of note, there is increased recognition of older individuals with HCM at advanced ages of >60 years with mild disease progression. Despite the similar prevalence regardless of ethnicity, some differences are worth mentioning in Asian HCM populations; for instance, the apical type of HCM, which is associated with a relatively benign course, is more common in around 30% of Asians,9)10)11) compared to 7% in Caucasians.12)13) This may partly account for the relatively lower incidence of obstructive physiology observed in Asian HCM.9)11)
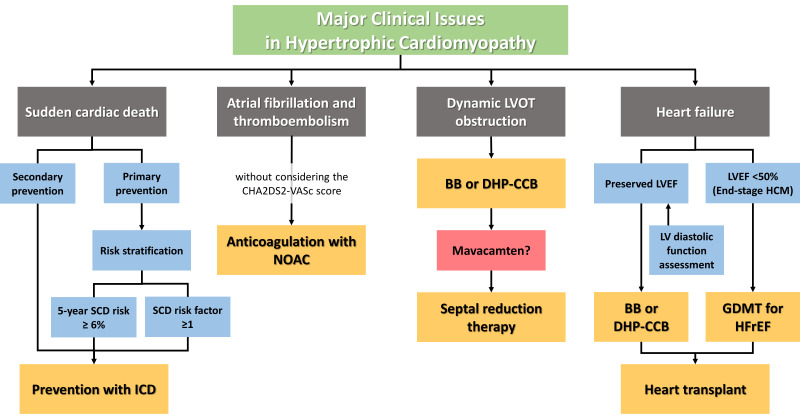
While many patients with HCM can expect a normal life expectancy without disabling symptoms or the need for major treatment, approximately one-third of patients with HCM experience disease-related complications,14)15) such as sudden cardiac death (SCD) due to malignant ventricular arrhythmia, symptoms related to dynamic LVOT obstruction, development of atrial fibrillation (AF) with related thromboembolic stroke, and heart failure (HF) due to advanced LV diastolic dysfunction with or without systolic dysfunction. Despite contemporary management strategies, HCM patients are still at increased risk for HCM-related and all-cause mortality compared to the general population,16) and more than one-fifth of HCM patients visit the emergency department during a 1-year period.17) With this background, this review focuses on the current management of major clinical issues in patients with HCM, including SCD, AF, LVOT obstruction, and HF.
Until the last part of the 20th century, there were no effective management strategies to prevent SCD in patients with HCM. Prophylactic administration of beta-blockers, calcium-channel blockers, and/or antiarrhythmic drugs was done with the “positive” hope of SCD prevention, without any solid evidence.18) Since then, the possibility of implantable cardioverter-defibrillators (ICD) placement for aborting fatal ventricular arrhythmias in humans was first suggested in 1980.19) Subsequently, ICDs were proven to be effective for the primary and secondary prevention of SCD in high-risk patients with HCM in 2000,20) with ventricular tachycardia or fibrillation shown to be the principal mechanism of SCD in these patients. The introduction of ICD has revolutionized the prevention of SCD in patients with HCM, and life-saving appropriate ICD therapy rates have been reported to be approximately 10%/year for secondary prevention and 4%/year for primary prevention in retrospective HCM registries.18)20)21) However, ICD implantation is not without its risks, as a substantial number of ICD-related issues have been reported, with a 4.8% annual rate of inappropriate ICD intervention and 3.4% annual rate of ICD-related complications.21) Thus, the risks and benefits of ICD placement should be carefully weighed and discussed in depth with patients and family members before the final decision. There is no disagreement that ICDs should be implanted for secondary prevention in HCM patients who have aborted SCD, ventricular fibrillation, or sustained ventricular tachycardia; however, in a primary prevention setting, different risk stratification strategies have evolved to select patients at high risk of SCD who would benefit from ICD placement.3)22)
The 2014 European Society of Cardiology (ESC) guidelines for HCM recommend using the HCM Risk-SCD model23) to estimate the 5-year risk of SCD.22) The variables included in the HCM Risk-SCD model are age, family history of SCD, unexplained syncope, LVOT gradient (at rest or with Valsalva maneuver), maximum LV wall thickness, left atrial (LA) dimension, and non-sustained ventricular tachycardia on Holter monitoring. The result is a continuous estimate of the 5-year SCD risk. ICD implantation is recommended in patients with a 5-year SCD risk ≥6% (class IIa) and may be considered in patients with a 5-year SCD risk ≥4% and <6% (class IIb); however, it is not recommended in patients with a 5-year SCD risk <4%. The ESC 5-year SCD risk model was validated in the Korean HCM population.24)
In contrast, the American Heart Association (AHA)/American College of Cardiology (ACC) guidelines recommend the use of a risk factor-based strategy for ICD patient selection. Patients with ≥1 established risk factor are considered candidates for ICD implantation. In the 2011 ACC/AHA guidelines, established SCD risk factors included family history of SCD, unexplained syncope, maximum LV wall thickness ≥30 mm (class IIa), non-sustained ventricular tachycardia on Holter monitoring, and abnormal blood pressure response during exercise (class IIb).25) In the recently revised 2020 AHA/ACC guidelines, abnormal blood pressure response was removed, while apical aneurysm, left ventricular ejection fraction (LVEF) <50% (class IIa), and extensive late gadolinium enhancement (LGE) on cardiac magnetic resonance (CMR) imaging (class IIb) were formally included in the SCD risk factors.3) In contrast to the ESC guidelines, LVOT gradient and left atrial diameter were not included in SCD risk evaluation in the 2020 AHA/ACC guidelines.
Before the revised 2020 AHA/ACC guidelines for HCM were published, LV systolic dysfunction was not one of the established major SCD risk factors considered for ICD placement. In HCM, LV systolic dysfunction is defined with the cutoff of LVEF <50%,3)22) which is a higher threshold than that of LVEF ≤40%, a threshold used to define HF with reduced ejection fraction in the general population.26)27) Clinically, the threshold of 50% is of vital importance given that LVEF <50% was reported to be associated with an increased risk of SCD in HCM.28)29)30) Consequently, LVEF <50% is now included as an established SCD risk factor in the 2020 AHA/ACC guidelines with a class IIa recommendation.
Although LVEF is a well-established index for LV systolic function, the LVEF is an insensitive index and thus is decreased only at the later stage of LV systolic dysfunction in HCM.31) To overcome this pitfall of LVEF, the left ventricular global longitudinal strain (LV-GLS) has been introduced as a new, sensitive index for detecting early LV systolic dysfunction.32) This index may thus be more useful in the assessment of HCM, as around 90–95% of HCM patients have preserved LVEF.29)30) Moreover, LV-GLS also reflects LV hypertrophy, myofiber disarray, and myocardial fibrosis in HCM.33)34) With this background, decreased absolute LV-GLS <15% has been shown to be associated with an increased risk of SCD events.35)36) Moreover, the addition of LV-GLS to the ESC risk score or the 2020 AHA/ACC risk factor strategy enhanced the performance of both models for predicting SCD events, suggesting the potential of LV-GLS for incorporation in the SCD risk prediction algorithms.35) LV-GLS may be especially useful for improving the sensitivity of the ESC model and the specificity of the AHA/ACC model.35)37)38)
LGE on CMR imaging is a noninvasive representation of irreversible replacement fibrosis of the myocardium, which may act as a structural nidus for lethal ventricular arrhythmias.39) LGE in HCM is usually measured in a quantitative manner using the threshold of 6 standard deviations above the mean signal intensity of the normal myocardium in HCM.40) LGE is present in approximately 50–80% of patients with HCM41)42)43) and can progress with time.44) The extent of LGE was reported to be proportionally associated with ventricular tachyarrhythmia events, including non-sustained ventricular tachycardia.43) The increasing extent of LGE was shown to be associated with a higher incidence of SCD events, and LGE ≥15% has been proposed as a threshold for high SCD risk.41)42)45) However, LGE quantification may differ according to the method used, and no general consensus on the optimal threshold has been made.
AF is commonly observed in HCM, with an estimated prevalence of approximately 20% in referral-based cohorts.46) AF development in HCM is associated with multiple factors, including worse LV diastolic dysfunction, greater LA size, worse LA function, older age, female sex, worse functional class, and the presence of hypertension.47)48) From a clinical point of view, the presence of AF is associated with a substantially higher risk of stroke, HF, and death in HCM.49)50)51) In particular, AF is associated with a 60% higher risk of stroke in patients with HCM, and this AF-associated stroke risk is augmented even in patients with no or few risk factors.51) As AF-associated stroke risk in HCM is significantly increased irrespective of the CHA2DS2-VASc score, aggressive intervention for stroke prevention is important. With the use of contemporary treatment strategies for AF, including effective anticoagulation and rhythm control with antiarrhythmic drugs or AF ablation, the disease burden and fatal consequences of AF in HCM may be reduced to low levels similar to those in the general population.52)
Effective and safe anticoagulation is of particular importance in reducing the thromboembolic risk associated with AF in HCM.46)52)53) The CHA2DS2-VASc score, used for clinical decision of anticoagulation strategy in the general population with AF, showed poor prediction of stroke risk in HCM patients with AF, and even HCM patients with AF but no risk factors (i.e., CHA2DS2-VASc score=0) are at an elevated risk of thromboembolic events.51)53) Thus, anticoagulation should be strongly considered in HCM patients with AF regardless of the CHA2DS2-VASc score. According to the 2014 ESC guidelines and the 2011 ACC/AHA guidelines, all HCM patients with AF, either paroxysmal or persistent, were recommended to receive anticoagulation with vitamin K antagonists.22)25) Since then, non-vitamin K antagonist oral anticoagulants have been reported to show superior effectiveness and safety compared to vitamin K antagonists for the primary and secondary prevention of stroke in patients with HCM and AF54)55) and are recommended as the first-line option for anticoagulation in the 2020 AHA/ACC guidelines.3)
Beta-blockers and non-dihydropyridine calcium channel blockers, such as verapamil and diltiazem, are recommended for rate control in HCM patients with AF,3)22) which can also be effective for dynamic LVOT obstruction, if present. There is a lack of firm evidence regarding whether rhythm control is beneficial in HCM. Current guidelines recommend that a rhythm control strategy with antiarrhythmic drugs or catheter ablation can be considered when AF is poorly tolerated in HCM as a class IIa indication.3) Amiodarone and sotalol are the most commonly used antiarrhythmic drugs in HCM patients with AF.52) Meanwhile, data on class Ic antiarrhythmic drugs such as flecainide or propafenone are limited due to proarrhythmic safety concerns in patients with structural heart disease; thus, these drugs should not be used in the absence of an ICD. Successful rhythm control rates with catheter ablation are lower in HCM patients with AF than that in non-HCM patients with AF; however, catheter ablation is still an effective option, especially in patients with paroxysmal AF and small atria.56)57) Surgical AF ablation has been reported to be more effective than catheter ablation52) and can be considered concomitantly when there is a need for other surgical procedures such as myectomy.
In HCM, septal hypertrophy and anatomic alterations in the mitral valve apparatus causing systolic anterior motion of the mitral valve leaflets contribute to dynamic LVOT obstruction.58)59) Conventionally, obstructive physiology is defined as peak LVOT pressure gradient ≥30 mmHg at rest or with provocation; meanwhile, hemodynamically significant obstruction usually occurs at ≥50 mmHg.3)22) Around one-third of HCM patients have been reported to have dynamic LVOT obstruction at rest, and another one-third show latent obstruction that can be provoked with physiological maneuvers.60) The reported incidence of obstructive physiology was lower in Asian cohorts compared to that of Western cohorts, at around 10–15% at rest61)62) and around 20–30% even with utilization of the Valsalva maneuver for provocation.11)35) The greater prevalence of the apical subtype of HCM in Asians may contribute to these observations.9) Increased myocardial contractility, smaller LV cavity size due to decreased preload, and decreased afterload can aggravate dynamic LVOT obstruction.
Drugs that decrease myocardial contractility (beta-blockers, non-dihydropyridine calcium channel blockers, and disopyramide) have been used empirically to improve the symptoms of LVOT obstruction in HCM. Current guidelines recommend maximally tolerated non-vasodilating beta-blockers or non-dihydropyridine calcium channel blockers (e.g., verapamil, diltiazem) as first-line therapy for symptomatic obstructive HCM.3)22) Whether using a combination of both drugs can be beneficial is uncertain.3) For patients who continue to have severe symptoms related to LVOT obstruction, the addition of disopyramide is recommended; however, this drug is currently unavailable in Korea. However, these drugs are often inadequate or poorly tolerated. If symptoms of LVOT obstruction are not controlled with medication, the next step is to consider septal reduction therapy, by either septal myectomy or alcohol septal ablation, performed at experienced centers.3)22)
In April 2022, the US Food and Drug Administration approved mavacamten for the treatment of symptomatic obstructive HCM. Mavacamten is an allosteric inhibitor of cardiac myosin, which decreases actin–myosin cross-bridge formation, thereby reducing sarcomere hypercontractility inherent to HCM and improving myocardial energetics. Treatment with mavacamten in obstructive HCM patients with New York Heart Association class II to III symptoms led to marked improvement in LVOT obstruction, subjective symptoms, and exercise capacity.63)64) Mavacamten was also suggested to be associated with the reduction of pathological LV hypertrophy in HCM.65) The majority of pathogenic genetic variants in HCM occur in the genes that encode the cardiac myosin or myosin-binding protein, which likely lead to changes in the myocardial energetics causing hypercontractility and, finally, myocyte hypertrophy and fibrosis.66) Thus, mavacamten, which targets the fundamental pathophysiology of HCM, may open a new chapter in HCM management.
In patients with nonobstructive type of HCM, HF more commonly takes the form of HF with preserved ejection rather than reduced ejection fraction. Current guidelines recommend the use of beta-blockers or non-dihydropyridine calcium channel blockers and cautious use of diuretics to control symptoms of dyspnea in patients with nonobstructive HCM and preserved ejection fraction, under the idea that the negative inotropic and chronotropic effect of these drugs will reduce LV filling pressures and myocardial oxygen demand.3)22) LV diastolic dysfunction with or without LVOT dynamic obstruction is the main pathophysiological mechanism that causes symptoms of dyspnea and HF events in these patients with HF with preserved ejection fraction. Myocardial hypertrophy, ischemia, and fibrosis, as well as impaired cardiac energetics inherent to HCM, contribute to impaired myocardial relaxation, chamber stiffness, and advanced diastolic dysfunction.3)67)68)69)70) Consequently, evaluation of LV filling pressure and the state of diastolic dysfunction in these patients can be crucial for deciding management strategies, but this is in many cases highly problematic.
Notably, noninvasive echocardiographic evaluation of LV diastolic function in HCM is complex and not well-established.71) Individual echocardiographic parameters showed, at best, modest correlations with LV filling pressures in HCM, which may be related to the heterogeneity in hypertrophic LV segments, myocyte disarray, myocardial fibrosis, and ischemia.71) The ratio of early mitral inflow velocity to early mitral annular velocity (E/e′ ratio) was only modestly correlated with invasively measured mean LA pressure in HCM (r=0.44, p<0.001); more importantly, individual values were widely scattered, and up to 25% of patients with an E/e′ ratio of >15 had no elevation in mean LA pressure.72) Also, LA volume index showed poor correlation with mean LA pressure and LV end-diastolic pressure in HCM.73) Thus, the current guidelines recommend that a combination of echocardiographic parameters such as the E/e′ ratio, LA volume index, pulmonary vein atrial reversal velocity, maximum tricuspid regurgitation velocity, the ratio of early to late mitral inflow velocity (E/A), and e′ velocity should be considered in the grading of LV diastolic function in HCM.71) However, these parameters are often discrepant and can lead to indeterminate results. LA mechanics are affected by increased LV filling pressure, which is associated with LV diastolic dysfunction. An increase in LA volume reflects chronic elevation of LV filling pressure and is considered one of the parameters of LV diastolic function.74) Recent studies have shown that LA function measured by LA strain, especially reservoir strain, reflects elevated LV filling pressure as well as early LV diastolic dysfunction.75)76)77) As LA reservoir strain has been shown to have substantial value for assessing LV diastolic function, it has been suggested to be included in the algorithm for estimating LV filling pressure.78) In HCM, LA reservoir strain reflected the stage of LV diastolic dysfunction, and was also a strong predictor of future HF events.79) Based on these data, the incorporation of LA reservoir strain into the algorithms assessing LV diastolic function in HCM should be considered.80)
The term “end-stage” HCM has been used clinically to describe patients with advanced LV systolic dysfunction, defined as an LVEF of <50%. According to the current evidence, approximately 5% of HCM patients have reduced LVEF.28)29)30) Up to 50% of these HCM patients develop refractory HF, leading to death, heart transplantation, or LV assist device implantation.29)30) Advanced HF in HCM with uncontrolled symptoms despite guideline-directed maximal medical treatment is often associated with reduced LVEF, but can also occur in patients with advanced LV diastolic dysfunction.81) Advanced HF in HCM is frequently accompanied by diffuse replacement myocardial fibrosis, i.e., LV scarring.82) Replacement myocardial fibrosis that can be quantified by LGE on CMR appears to be progressive in HCM,44) and greater progression is associated with progression to LVEF <50% and admission for HF.70)83) There were reports suggesting that LGE confined to right ventricular insertion points in HCM is also associated with worse LV diastolic and systolic function84) and may be an intermediate stage between patients with no LGE and patients with intramural LGE.85) Predictors of progression to “end-stage” HCM include greater LV size and wall thickness, borderline LVEF of 50% to 60%, the presence of LGE, and the presence of a pathogenic sarcomeric gene variant.30) Patients with HCM who develop LVEF <50% should receive optimal guideline-directed medical therapy for HF with reduced ejection fraction and should be considered candidates for device therapy including ICD and cardiac resynchronization therapy.3) Moreover, discontinuation of negative inotropic agents such as verapamil and diltiazem, or a change to beta-blockers, if necessary, should be considered. Heart transplantation should also be considered in HCM patients with refractory advanced HF.86)
Other comorbidities contributing to HF in HCM should also be evaluated and aggressively managed. AF is an important contributor to HF progression in HCM, with the loss of effective atrial contraction and uncontrolled tachycardia; in addition, AF itself can be a consequence of chronically elevated LA pressure related to HF.30)87)88) Diabetes mellitus, which often coexists in elderly HCM patients, is associated with a higher risk of HF and mortality, possibly related to the combined effect of HCM and diabetes on the myocardium, and should be actively managed.89)90) Female sex is also reported to be an independent risk factor for HF,91) the effect of which appears to become more prominent at older age/postmenopause.92) Lastly, epicardial coronary artery disease should be considered as a cause of exertional dyspnea or chest pain in patients with HCM, and should be managed aggressively given its prognostic impact.93)94)
By actively implementing contemporary management strategies in HCM, morbidity and mortality can be substantially reduced. SCD in HCM can be effectively prevented with ICDs; however, appropriate patient selection is important, especially for primary prevention. There is no doubt that ICDs should be implanted for the secondary prevention of SCD in HCM patients with aborted SCD events or sustained ventricular tachycardia. For the primary prevention of SCD in HCM, the 5-year SCD risk score calculated according to the 2014 ESC guidelines and the presence of major SCD risk factors according to the 2020 AHA/ACC guidelines should be considered. LGE on CMR and LV-GLS on echocardiography can provide an additive value for SCD risk prediction. AF is an important contributor to morbidity and mortality in patients with HCM, and the importance of effective anticoagulation cannot be overemphasized. Non-vitamin K antagonist oral anticoagulants should be a first option in all HCM patients with AF, regardless of the CHA2DS2-VASc score. Dynamic LVOT obstruction causing symptoms is first treated medically with beta-blockers or non-dihydropyridine calcium channel blockers, and if symptoms persist, septal reduction therapy is considered. Mavacamten has been approved for the treatment of symptomatic obstructive HCM and may open a new chapter in HCM management. HF in HCM is usually attributed to LV diastolic dysfunction. LA strain is useful for assessing LV diastolic function in HCM independent of other established echocardiographic markers. Approximately 5% of HCM patients show reduced LVEF <50%, also referred to as “end-stage” HCM. Myocardial fibrosis plays an important role in the progression to advanced HF in patients with HCM. Although echocardiography and CMR currently play an essential and complementary role in the management of HCM, further studies are needed to establish how myocardial deformation and LGE can provide better risk stratification and guide treatment.
Notes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest: HKK reports research grants from Actelion, Handok, GSK, Dae-Woong, Hanmi, Ildong, JW Pharmaceutical, Samjin, and Norvatis. The remaining authors declare no conflict of interest.
References
1. Teare D. Asymmetrical hypertrophy of the heart in young adults. Br Heart J. 1958; 20:1–8. PMID: 13499764.

2. Morrow AG, Braunwald E. Functional aortic stenosis; a malformation characterized by resistance to left ventricular outflow without anatomic obstruction. Circulation. 1959; 20:181–189. PMID: 13671704.
3. Ommen SR, Mital S, Burke MA, et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2020; 142:e558–e631. PMID: 33215931.
4. Ho CY, Day SM, Ashley EA, et al. Genotype and lifetime burden of disease in hypertrophic cardiomyopathy: insights from the Sarcomeric Human Cardiomyopathy Registry (SHaRe). Circulation. 2018; 138:1387–1398. PMID: 30297972.

5. Kim KH, Pereira NL. Genetics of cardiomyopathy: clinical and mechanistic implications for heart failure. Korean Circ J. 2021; 51:797–836. PMID: 34327881.

6. Semsarian C, Ingles J, Maron MS, Maron BJ. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2015; 65:1249–1254. PMID: 25814232.
7. Maron MS, Hellawell JL, Lucove JC, Farzaneh-Far R, Olivotto I. Occurrence of clinically diagnosed hypertrophic cardiomyopathy in the United States. Am J Cardiol. 2016; 117:1651–1654. PMID: 27006153.

8. Moon I, Lee SY, Kim HK, et al. Trends of the prevalence and incidence of hypertrophic cardiomyopathy in Korea: a nationwide population-based cohort study. PLoS One. 2020; 15:e0227012. PMID: 31929538.

9. Kitaoka H, Doi Y, Casey SA, Hitomi N, Furuno T, Maron BJ. Comparison of prevalence of apical hypertrophic cardiomyopathy in Japan and the United States. Am J Cardiol. 2003; 92:1183–1186. PMID: 14609593.

10. Kubo T, Kitaoka H, Okawa M, et al. Clinical profiles of hypertrophic cardiomyopathy with apical phenotype--comparison of pure-apical form and distal-dominant form. Circ J. 2009; 73:2330–2336. PMID: 19838003.

11. Kim EK, Lee SC, Hwang JW, et al. Differences in apical and non-apical types of hypertrophic cardiomyopathy: a prospective analysis of clinical, echocardiographic, and cardiac magnetic resonance findings and outcome from 350 patients. Eur Heart J Cardiovasc Imaging. 2016; 17:678–686. PMID: 26245912.

12. Eriksson MJ, Sonnenberg B, Woo A, et al. Long-term outcome in patients with apical hypertrophic cardiomyopathy. J Am Coll Cardiol. 2002; 39:638–645. PMID: 11849863.

13. Klarich KW, Attenhofer Jost CH, Binder J, et al. Risk of death in long-term follow-up of patients with apical hypertrophic cardiomyopathy. Am J Cardiol. 2013; 111:1784–1791. PMID: 23540548.

14. Maron BJ, Casey SA, Hauser RG, Aeppli DM. Clinical course of hypertrophic cardiomyopathy with survival to advanced age. J Am Coll Cardiol. 2003; 42:882–888. PMID: 12957437.

15. Rowin EJ, Maron MS, Chan RH, et al. Interaction of adverse disease related pathways in hypertrophic cardiomyopathy. Am J Cardiol. 2017; 120:2256–2264. PMID: 29111210.

16. Kwon S, Kim HK, Kim B, et al. Comparison of mortality and cause of death between adults with and without hypertrophic cardiomyopathy. Sci Rep. 2022; 12:6386. PMID: 35430580.

17. Choi YJ, Kim B, Lee HJ, et al. Emergency department utilization in patients with hypertrophic cardiomyopathy: a nationwide population-based study. Sci Rep. 2022; 12:3534. PMID: 35241727.

18. Maron BJ, Maron MS. Contemporary strategies for risk stratification and prevention of sudden death with the implantable defibrillator in hypertrophic cardiomyopathy. Heart Rhythm. 2016; 13:1155–1165. PMID: 26749314.

19. Mirowski M, Reid PR, Mower MM, et al. Termination of malignant ventricular arrhythmias with an implanted automatic defibrillator in human beings. N Engl J Med. 1980; 303:322–324. PMID: 6991948.

20. Maron BJ, Shen WK, Link MS, et al. Efficacy of implantable cardioverter-defibrillators for the prevention of sudden death in patients with hypertrophic cardiomyopathy. N Engl J Med. 2000; 342:365–373. PMID: 10666426.

21. Schinkel AF, Vriesendorp PA, Sijbrands EJ, Jordaens LJ, ten Cate FJ, Michels M. Outcome and complications after implantable cardioverter defibrillator therapy in hypertrophic cardiomyopathy: systematic review and meta-analysis. Circ Heart Fail. 2012; 5:552–559. PMID: 22821634.

22. Authors/Task Force members. Elliott PM, Anastasakis A, et al. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the task force for the diagnosis and management of hypertrophic cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014; 35:2733–2779. PMID: 25173338.

23. O’Mahony C, Jichi F, Pavlou M, et al. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM risk-SCD). Eur Heart J. 2014; 35:2010–2020. PMID: 24126876.

24. Choi YJ, Kim HK, Lee SC, et al. Validation of the hypertrophic cardiomyopathy risk-sudden cardiac death calculator in Asians. Heart. 2019; 105:1892–1897. PMID: 31383719.

25. Gersh BJ, Maron BJ, Bonow RO, et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011; 124:e783–e831. PMID: 22068434.
26. McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021; 42:3599–3726. PMID: 34447992.
27. Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022; 145:e895–1032. PMID: 35363499.
28. Harris KM, Spirito P, Maron MS, et al. Prevalence, clinical profile, and significance of left ventricular remodeling in the end-stage phase of hypertrophic cardiomyopathy. Circulation. 2006; 114:216–225. PMID: 16831987.

29. Rowin EJ, Maron BJ, Carrick RT, et al. Outcomes in patients with hypertrophic cardiomyopathy and left ventricular systolic dysfunction. J Am Coll Cardiol. 2020; 75:3033–3043. PMID: 32553256.

30. Marstrand P, Han L, Day SM, et al. Hypertrophic cardiomyopathy with left ventricular systolic dysfunction: insights from the SHaRe Registry. Circulation. 2020; 141:1371–1383. PMID: 32228044.

31. Olivotto I, Cecchi F, Poggesi C, Yacoub MH. Patterns of disease progression in hypertrophic cardiomyopathy: an individualized approach to clinical staging. Circ Heart Fail. 2012; 5:535–546. PMID: 22811549.
32. Park JH. Two-dimensional echocardiographic assessment of myocardial strain: important echocardiographic parameter readily useful in clinical field. Korean Circ J. 2019; 49:908–931. PMID: 31456367.

33. Kobayashi T, Popovic Z, Bhonsale A, et al. Association between septal strain rate and histopathology in symptomatic hypertrophic cardiomyopathy patients undergoing septal myectomy. Am Heart J. 2013; 166:503–511. PMID: 24016500.

34. Urbano-Moral JA, Rowin EJ, Maron MS, Crean A, Pandian NG. Investigation of global and regional myocardial mechanics with 3-dimensional speckle tracking echocardiography and relations to hypertrophy and fibrosis in hypertrophic cardiomyopathy. Circ Cardiovasc Imaging. 2014; 7:11–19. PMID: 24275954.

35. Lee HJ, Kim HK, Lee SC, et al. Supplementary role of left ventricular global longitudinal strain for predicting sudden cardiac death in hypertrophic cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2022; 23:1108–1116. PMID: 34542591.

36. Hiemstra YL, Debonnaire P, Bootsma M, et al. Global longitudinal strain and left atrial volume index provide incremental prognostic value in patients with hypertrophic cardiomyopathy. Circ Cardiovasc Imaging. 2017; 10:e005706. PMID: 28679523.

37. Udelson JE. Evaluating and reducing the risk of sudden death in hypertrophic cardiomyopathy. Circulation. 2019; 139:727–729. PMID: 30715941.

38. Wang J, Zhang Z, Li Y, Xu Y, Wan K, Chen Y. Variable and limited predictive value of the European Society of Cardiology hypertrophic cardiomyopathy sudden-death risk model: a meta-analysis. Can J Cardiol. 2019; 35:1791–1799. PMID: 31474312.

39. Cho JH. Sudden death and ventricular arrhythmias in heart failure with preserved ejection fraction. Korean Circ J. 2022; 52:251–264. PMID: 35388994.

40. Harrigan CJ, Peters DC, Gibson CM, et al. Hypertrophic cardiomyopathy: quantification of late gadolinium enhancement with contrast-enhanced cardiovascular MR imaging. Radiology. 2011; 258:128–133. PMID: 21045187.

41. Chan RH, Maron BJ, Olivotto I, et al. Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation. 2014; 130:484–495. PMID: 25092278.

42. Weng Z, Yao J, Chan RH, et al. Prognostic value of LGE-CMR in HCM: a meta-analysis. JACC Cardiovasc Imaging. 2016; 9:1392–1402. PMID: 27450876.
43. Park YJ, Park SJ, Kim EK, et al. Semi-quantitative versus quantitative assessments of late gadolinium enhancement extent for predicting spontaneous ventricular tachyarrhythmia events in patients with hypertrophic cardiomyopathy. Sci Rep. 2020; 10:2920. PMID: 32076039.

44. Choi HM, Kim KH, Lee JM, et al. Myocardial fibrosis progression on cardiac magnetic resonance in hypertrophic cardiomyopathy. Heart. 2015; 101:870–876. PMID: 25897040.

45. Mentias A, Raeisi-Giglou P, Smedira NG, et al. Late gadolinium enhancement in patients with hypertrophic cardiomyopathy and preserved systolic function. J Am Coll Cardiol. 2018; 72:857–870. PMID: 30115224.

46. Guttmann OP, Rahman MS, O’Mahony C, Anastasakis A, Elliott PM. Atrial fibrillation and thromboembolism in patients with hypertrophic cardiomyopathy: systematic review. Heart. 2014; 100:465–472. PMID: 24014282.

47. Guttmann OP, Pavlou M, O’Mahony C, et al. Predictors of atrial fibrillation in hypertrophic cardiomyopathy. Heart. 2017; 103:672–678. PMID: 27794017.

48. Debonnaire P, Joyce E, Hiemstra Y, et al. Left atrial size and function in hypertrophic cardiomyopathy patients and risk of new-onset atrial fibrillation. Circ Arrhythm Electrophysiol. 2017; 10:e004052. PMID: 28183843.

49. Olivotto I, Cecchi F, Casey SA, Dolara A, Traverse JH, Maron BJ. Impact of atrial fibrillation on the clinical course of hypertrophic cardiomyopathy. Circulation. 2001; 104:2517–2524. PMID: 11714644.

50. Siontis KC, Geske JB, Ong K, Nishimura RA, Ommen SR, Gersh BJ. Atrial fibrillation in hypertrophic cardiomyopathy: prevalence, clinical correlations, and mortality in a large high-risk population. J Am Heart Assoc. 2014; 3:e001002. PMID: 24965028.

51. Lee HJ, Kim HK, Kim M, et al. Clinical impact of atrial fibrillation in a nationwide cohort of hypertrophic cardiomyopathy patients. Ann Transl Med. 2020; 8:1386. PMID: 33313131.

52. Rowin EJ, Hausvater A, Link MS, et al. Clinical Profile and Consequences of Atrial Fibrillation in Hypertrophic Cardiomyopathy. Circulation. 2017; 136:2420–2436. PMID: 28916640.

53. Guttmann OP, Pavlou M, O’Mahony C, et al. Prediction of thrombo-embolic risk in patients with hypertrophic cardiomyopathy (HCM Risk-CVA). Eur J Heart Fail. 2015; 17:837–845. PMID: 26183688.

54. Lee HJ, Kim HK, Jung JH, et al. Novel oral anticoagulants for primary stroke prevention in hypertrophic cardiomyopathy patients with atrial fibrillation. Stroke. 2019; 50:2582–2586. PMID: 31340730.

55. Jung H, Yang PS, Jang E, et al. Effectiveness and safety of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation with hypertrophic cardiomyopathy: a nationwide cohort study. Chest. 2019; 155:354–363. PMID: 30472021.

56. Providencia R, Elliott P, Patel K, et al. Catheter ablation for atrial fibrillation in hypertrophic cardiomyopathy: a systematic review and meta-analysis. Heart. 2016; 102:1533–1543. PMID: 27234160.

57. Zhao DS, Shen Y, Zhang Q, et al. Outcomes of catheter ablation of atrial fibrillation in patients with hypertrophic cardiomyopathy: a systematic review and meta-analysis. Europace. 2016; 18:508–520. PMID: 26612881.

58. Kim DH, Handschumacher MD, Levine RA, et al. In vivo measurement of mitral leaflet surface area and subvalvular geometry in patients with asymmetrical septal hypertrophy: insights into the mechanism of outflow tract obstruction. Circulation. 2010; 122:1298–1307. PMID: 20837895.

59. Patel P, Dhillon A, Popovic ZB, et al. Left ventricular outflow tract obstruction in hypertrophic cardiomyopathy patients without severe septal hypertrophy: implications of mitral valve and papillary muscle abnormalities assessed using cardiac magnetic resonance and echocardiography. Circ Cardiovasc Imaging. 2015; 8:e003132. PMID: 26082555.

60. Maron MS, Olivotto I, Zenovich AG, et al. Hypertrophic cardiomyopathy is predominantly a disease of left ventricular outflow tract obstruction. Circulation. 2006; 114:2232–2239. PMID: 17088454.

61. Kubo T, Hirota T, Baba Y, et al. Patients’ characteristics and clinical course of hypertrophic cardiomyopathy in a regional Japanese cohort - results from Kochi RYOMA study. Circ J. 2018; 82:824–830. PMID: 29332907.

62. Ho HH, Lee KL, Lau CP, Tse HF. Clinical characteristics of and long-term outcome in Chinese patients with hypertrophic cardiomyopathy. Am J Med. 2004; 116:19–23. PMID: 14706661.

63. Olivotto I, Oreziak A, Barriales-Villa R, et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2020; 396:759–769. PMID: 32871100.
64. Spertus JA, Fine JT, Elliott P, et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): health status analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021; 397:2467–2475. PMID: 34004177.

65. Saberi S, Cardim N, Yamani M, et al. Mavacamten favorably impacts cardiac structure in obstructive hypertrophic cardiomyopathy: EXPLORER-HCM cardiac magnetic resonance substudy analysis. Circulation. 2021; 143:606–608. PMID: 33190524.

66. Elliott PM. The end of the beginning for drug therapy in obstructive hypertrophic cardiomyopathy with EXPLORER-HCM. Cardiovasc Res. 2020; 116:e175–e178. PMID: 33096564.

67. Ahn HS, Kim HK, Park EA, et al. Coronary flow reserve impairment in apical vs asymmetrical septal hypertrophic cardiomyopathy. Clin Cardiol. 2013; 36:207–216. PMID: 23378014.

68. Kim EK, Lee SC, Chang SA, et al. Prevalence and clinical significance of cardiovascular magnetic resonance adenosine stress-induced myocardial perfusion defect in hypertrophic cardiomyopathy. J Cardiovasc Magn Reson. 2020; 22:30. PMID: 32366254.

69. Dass S, Cochlin LE, Suttie JJ, et al. Exacerbation of cardiac energetic impairment during exercise in hypertrophic cardiomyopathy: a potential mechanism for diastolic dysfunction. Eur Heart J. 2015; 36:1547–1554. PMID: 25990345.

70. Raphael CE, Mitchell F, Kanaganayagam GS, et al. Cardiovascular magnetic resonance predictors of heart failure in hypertrophic cardiomyopathy: the role of myocardial replacement fibrosis and the microcirculation. J Cardiovasc Magn Reson. 2021; 23:26. PMID: 33685501.

71. Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016; 29:277–314. PMID: 27037982.

72. Geske JB, Sorajja P, Nishimura RA, Ommen SR. Evaluation of left ventricular filling pressures by Doppler echocardiography in patients with hypertrophic cardiomyopathy: correlation with direct left atrial pressure measurement at cardiac catheterization. Circulation. 2007; 116:2702–2708. PMID: 18025528.

73. Geske JB, Sorajja P, Nishimura RA, Ommen SR. The relationship of left atrial volume and left atrial pressure in patients with hypertrophic cardiomyopathy: an echocardiographic and cardiac catheterization study. J Am Soc Echocardiogr. 2009; 22:961–966. PMID: 19524402.

74. Thomas L, Marwick TH, Popescu BA, Donal E, Badano LP. Left atrial structure and function, and left ventricular diastolic dysfunction: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019; 73:1961–1977. PMID: 31000000.

75. Huynh QL, Kalam K, Iannaccone A, Negishi K, Thomas L, Marwick TH. Functional and anatomic responses of the left atrium to change in estimated left ventricular filling pressure. J Am Soc Echocardiogr. 2015; 28:1428–1433.e1. PMID: 26343250.

76. Singh A, Addetia K, Maffessanti F, Mor-Avi V, Lang RM. LA strain for categorization of LV diastolic dysfunction. JACC Cardiovasc Imaging. 2017; 10:735–743. PMID: 28017389.
77. Morris DA, Belyavskiy E, Aravind-Kumar R, et al. Potential usefulness and clinical relevance of adding left atrial strain to left atrial volume index in the detection of left ventricular diastolic dysfunction. JACC Cardiovasc Imaging. 2018; 11:1405–1415. PMID: 29153567.

78. Smiseth OA, Morris DA, Cardim N, et al. Multimodality imaging in patients with heart failure and preserved ejection fraction: an expert consensus document of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2022; 23:e34–e61. PMID: 34729586.

79. Lee HJ, Kim HK, Rhee TM, et al. Left atrial reservoir strain-based left ventricular diastolic function grading and incident heart failure in hypertrophic cardiomyopathy. Circ Cardiovasc Imaging. 2022; 15:e013556. PMID: 35439039.

80. Oh JK, Miranda WR. Left atrial reservoir strain: a savior to diastolic function assessment in hypertrophic cardiomyopathy? Circ Cardiovasc Imaging. 2022; 15:e014148. PMID: 35439040.

81. Pasqualucci D, Fornaro A, Castelli G, et al. Clinical spectrum, therapeutic options, and outcome of advanced heart failure in hypertrophic cardiomyopathy. Circ Heart Fail. 2015; 8:1014–1021. PMID: 26446673.

82. Galati G, Leone O, Pasquale F, et al. Histological and histometric characterization of myocardial fibrosis in end-stage hypertrophic cardiomyopathy: a clinical-pathological study of 30 explanted hearts. Circ Heart Fail. 2016; 9:e003090. PMID: 27618852.

83. Habib M, Adler A, Fardfini K, et al. Progression of myocardial fibrosis in hypertrophic cardiomyopathy: a cardiac magnetic resonance study. JACC Cardiovasc Imaging. 2021; 14:947–958. PMID: 33248971.
84. Zhu Y, Park EA, Lee W, et al. Extent of late gadolinium enhancement at right ventricular insertion points in patients with hypertrophic cardiomyopathy: relation with diastolic dysfunction. Eur Radiol. 2015; 25:1190–1200. PMID: 25597022.

85. Bravo PE, Luo HC, Pozios I, et al. Late gadolinium enhancement confined to the right ventricular insertion points in hypertrophic cardiomyopathy: an intermediate stage phenotype? Eur Heart J Cardiovasc Imaging. 2016; 17:293–300. PMID: 26077330.

86. Mehra MR, Canter CE, Hannan MM, et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: a 10-year update. J Heart Lung Transplant. 2016; 35:1–23. PMID: 26776864.

87. Melacini P, Basso C, Angelini A, et al. Clinicopathological profiles of progressive heart failure in hypertrophic cardiomyopathy. Eur Heart J. 2010; 31:2111–2123. PMID: 20513729.

88. Anter E, Jessup M, Callans DJ. Atrial fibrillation and heart failure: treatment considerations for a dual epidemic. Circulation. 2009; 119:2516–2525. PMID: 19433768.
89. Lee HJ, Kim HK, Kim BS, et al. Impact of diabetes mellitus on the outcomes of subjects with hypertrophic cardiomyopathy: a nationwide cohort study. Diabetes Res Clin Pract. 2022; 186:109838. PMID: 35314254.

90. Wasserstrum Y, Barriales-Villa R, Fernández-Fernández X, et al. The impact of diabetes mellitus on the clinical phenotype of hypertrophic cardiomyopathy. Eur Heart J. 2019; 40:1671–1677. PMID: 30358878.

91. Kim M, Kim B, Choi YJ, et al. Sex differences in the prognosis of patients with hypertrophic cardiomyopathy. Sci Rep. 2021; 11:4854. PMID: 33649405.

92. Lee HJ, Kim HK, Lee SC, et al. Age-related sex differences in the outcomes of patients with hypertrophic cardiomyopathy. PLoS One. 2022; 17:e0264580. PMID: 35213653.

93. Sorajja P, Ommen SR, Nishimura RA, Gersh BJ, Berger PB, Tajik AJ. Adverse prognosis of patients with hypertrophic cardiomyopathy who have epicardial coronary artery disease. Circulation. 2003; 108:2342–2348. PMID: 14581405.

94. van der Velde N, Huurman R, Yamasaki Y, et al. Frequency and significance of coronary artery disease and myocardial bridging in patients with hypertrophic cardiomyopathy. Am J Cardiol. 2020; 125:1404–1412. PMID: 32111340.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download