1. Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. 2019; 139:e56–528. PMID:
30700139.
2. McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021; 42:3599–3726. PMID:
34447992.
3. Kaneko H, Itoh H, Yotsumoto H, et al. Association between the number of hospital admissions and in-hospital outcomes in patients with heart failure. Hypertens Res. 2020; 43:1385–1391. PMID:
32655133.

4. Cheng RK, Cox M, Neely ML, et al. Outcomes in patients with heart failure with preserved, borderline, and reduced ejection fraction in the Medicare population. Am Heart J. 2014; 168:721–730. PMID:
25440801.

5. Pandey A, Kitzman D, Whellan DJ, et al. Frailty among older decompensated heart failure patients: prevalence, association with patient-centered outcomes, and efficient detection methods. JACC Heart Fail. 2019; 7:1079–1088. PMID:
31779931.
6. Saitoh M, Takahashi Y, Okamura D, et al. Prognostic impact of hospital-acquired disability in elderly patients with heart failure. ESC Heart Fail. 2021; 8:1767–1774. PMID:
33838022.

7. Long L, Mordi IR, Bridges C, et al. Exercise-based cardiac rehabilitation for adults with heart failure. Cochrane Database Syst Rev. 2019; 1:CD003331. PMID:
30695817.

8. Taylor RS, Walker S, Smart NA, et al. Impact of exercise rehabilitation on exercise capacity and quality-of-life in heart failure: individual participant meta-analysis. J Am Coll Cardiol. 2019; 73:1430–1443. PMID:
30922474.
9. Coats AJ, Adamopoulos S, Radaelli A, et al. Controlled trial of physical training in chronic heart failure. Exercise performance, hemodynamics, ventilation, and autonomic function. Circulation. 1992; 85:2119–2131. PMID:
1591831.

10. Pearson MJ, Smart NA. Effect of exercise training on endothelial function in heart failure patients: a systematic review meta-analysis. Int J Cardiol. 2017; 231:234–243. PMID:
28089145.

11. Tu RH, Zeng ZY, Zhong GQ, et al. Effects of exercise training on depression in patients with heart failure: a systematic review and meta-analysis of randomized controlled trials. Eur J Heart Fail. 2014; 16:749–757. PMID:
24797230.

12. Haykowsky MJ, Liang Y, Pechter D, Jones LW, McAlister FA, Clark AM. A meta-analysis of the effect of exercise training on left ventricular remodeling in heart failure patients: the benefit depends on the type of training performed. J Am Coll Cardiol. 2007; 49:2329–2336. PMID:
17572248.

13. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA Guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Card Fail. 2017; 23:628–651. PMID:
28461259.

14. Makita S, Yasu T, Akashi Y, et al. JCS/JACR 2021 Guideline on rehabilitation in patients with cardiovascular disease. Circ J. 2021; [Epub ahead of print].
15. Fuentes-Abolafio IJ, Stubbs B, Pérez-Belmonte LM, Bernal-López MR, Gómez-Huelgas R, Cuesta-Vargas AI. Physical functional performance and prognosis in patients with heart failure: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2020; 20:512. PMID:
33297975.

16. Kitzman DW, Whellan DJ, Duncan P, et al. Physical rehabilitation for older patients hospitalized for heart failure. N Engl J Med. 2021; 385:203–216. PMID:
33999544.

17. Enright PL. The six-minute walk test. Respir Care. 2003; 48:783–785. PMID:
12890299.
18. Newman AB, Simonsick EM, Naydeck BL, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006; 295:2018–2026. PMID:
16670410.

19. Bittner V, Weiner DH, Yusuf S, et al. Prediction of mortality and morbidity with a 6-minute walk test in patients with left ventricular dysfunction. JAMA. 1993; 270:1702–1707. PMID:
8411500.

20. Metra M, Ponikowski P, Dickstein K, et al. Advanced chronic heart failure: a position statement from the Study Group on Advanced Heart Failure of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2007; 9:684–694. PMID:
17481947.

21. Fang JC, Ewald GA, Allen LA, et al. Advanced (stage D) heart failure: a statement from the Heart Failure Society of America Guidelines Committee. J Card Fail. 2015; 21:519–534. PMID:
25953697.

22. Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials. 1989; 10:407–415. PMID:
2691207.
23. Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006; 54:743–749. PMID:
16696738.

24. Singh SJ, Puhan MA, Andrianopoulos V, et al. An official systematic review of the European Respiratory Society/American Thoracic Society: measurement properties of field walking tests in chronic respiratory disease. Eur Respir J. 2014; 44:1447–1478. PMID:
25359356.

25. Frost AE, Langleben D, Oudiz R, et al. The 6-min walk test (6MW) as an efficacy endpoint in pulmonary arterial hypertension clinical trials: demonstration of a ceiling effect. Vascul Pharmacol. 2005; 43:36–39. PMID:
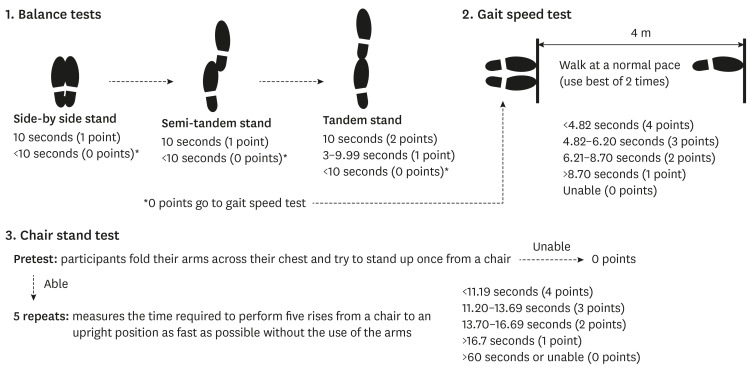
15890561.

26. Guazzi M, Adams V, Conraads V, et al. EACPR/AHA Joint Scientific Statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Eur Heart J. 2012; 33:2917–2927. PMID:
22952138.
27. Forman DE, Fleg JL, Kitzman DW, et al. 6-min walk test provides prognostic utility comparable to cardiopulmonary exercise testing in ambulatory outpatients with systolic heart failure. J Am Coll Cardiol. 2012; 60:2653–2661. PMID:
23177293.

28. Ueno K, Kamiya K, Hamazaki N, et al. Relationship between high-sensitivity cardiac troponin T, B-type natriuretic peptide, and physical function in patients with heart failure. ESC Heart Fail. 2021; 8:5092–5101. PMID:
34490747.

29. Ueno K, Kamiya K, Hamazaki N, et al. Usefulness of physical function sub-item of SF-36 survey to predict exercise intolerance in patients with heart failure. Eur J Cardiovasc Nurs. 2022; 21:174–177. PMID:
34324626.

30. Jourdain P, Funck F, Bellorini M, et al. Bedside B-type natriuretic peptide and functional capacity in chronic heart failure. Eur J Heart Fail. 2003; 5:155–160. PMID:
12644005.

31. Shah SJ, Cowie MR, Wachter R, et al. Baseline characteristics of patients in the PARALLAX trial: insights into quality of life and exercise capacity in heart failure with preserved ejection fraction. Eur J Heart Fail. 2021; 23:1541–1551. PMID:
34170062.

32. Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009; 13:881–889. PMID:
19924348.

33. Fritz S, Lusardi M. White paper: “walking speed: the sixth vital sign”. J Geriatr Phys Ther. 2009; 32:46–49. PMID:
20039582.

34. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011; 305:50–58. PMID:
21205966.

35. Montero-Odasso M, Schapira M, Soriano ER, et al. Gait velocity as a single predictor of adverse events in healthy seniors aged 75 years and older. J Gerontol A Biol Sci Med Sci. 2005; 60:1304–1309. PMID:
16282564.

36. Ostir GV, Kuo YF, Berges IM, Markides KS, Ottenbacher KJ. Measures of lower body function and risk of mortality over 7 years of follow-up. Am J Epidemiol. 2007; 166:599–605. PMID:
17566063.

37. Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003; 51:314–322. PMID:
12588574.

38. Chen LK, Woo J, Assantachai P, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020; 21:300–307.e2. PMID:
32033882.

39. Satake S, Shimada H, Yamada M, et al. Prevalence of frailty among community-dwellers and outpatients in Japan as defined by the Japanese version of the Cardiovascular Health Study criteria. Geriatr Gerontol Int. 2017; 17:2629–2634. PMID:
29265757.

40. Kim DH, Kim CA, Placide S, Lipsitz LA, Marcantonio ER. Preoperative frailty assessment and outcomes at 6 months or later in older adults undergoing cardiac surgical procedures: a systematic review. Ann Intern Med. 2016; 165:650–660. PMID:
27548070.

41. Afilalo J, Mottillo S, Eisenberg MJ, et al. Addition of frailty and disability to cardiac surgery risk scores identifies elderly patients at high risk of mortality or major morbidity. Circ Cardiovasc Qual Outcomes. 2012; 5:222–228. PMID:
22396586.

42. Pulignano G, Del Sindaco D, Di Lenarda A, et al. Incremental value of gait speed in predicting prognosis of older adults with heart failure: insights from the IMAGE-HF study. JACC Heart Fail. 2016; 4:289–298. PMID:
26970831.

43. Veronese N, Stubbs B, Volpato S, et al. Association between gait speed with mortality, cardiovascular disease and cancer: a systematic review and meta-analysis of prospective cohort studies. J Am Med Dir Assoc. 2018; 19:981–988.e7. PMID:
30056008.

44. Matsuzawa Y, Konishi M, Akiyama E, et al. Association between gait speed as a measure of frailty and risk of cardiovascular events after myocardial infarction. J Am Coll Cardiol. 2013; 61:1964–1972. PMID:
23500222.

45. Ueno K, Kamiya K, Hamazaki N, et al. Usefulness of measuring maximal gait speed in conjunction with usual gait speed for risk stratification in patients with cardiovascular disease. Exp Gerontol. 2022; 164:111810. PMID:
35452782.

46. Ueno K, Kaneko H, Kamiya K, et al. Clinical utility of simple subjective gait speed for the risk stratification of heart failure in a primary prevention setting. Sci Rep. 2022; 12:11641. PMID:
35803973.

47. Kaminsky LA, Tuttle MS. Functional assessment of heart failure patients. Heart Fail Clin. 2015; 11:29–36. PMID:
25432472.

48. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994; 49:M85–M94. PMID:
8126356.

49. Riskowski JL, Hagedorn TJ, Dufour AB, Hannan MT. Functional foot symmetry and its relation to lower extremity physical performance in older adults: the Framingham Foot Study. J Biomech. 2012; 45:1796–1802. PMID:
22560642.

50. European Medicines Agency (EMA). Reflection paper on physical frailty: instruments for baseline characterisation of older populations in clinical trials. London: European Medicines Agency;2018.
51. Subra J, Gillette-Guyonnet S, Cesari M, Oustric S, Vellas B. Platform Team. The integration of frailty into clinical practice: preliminary results from the Gérontopôle. J Nutr Health Aging. 2012; 16:714–720. PMID:
23076514.

52. Kitai T, Shimogai T, Tang WH, et al. Short physical performance battery vs. 6-minute walking test in hospitalized elderly patients with heart failure. European Heart Journal Open. 2021; 1:oeab006. PMID:
35919089.

53. Caetano MJ, Lord SR, Brodie MA, et al. Executive functioning, concern about falling and quadriceps strength mediate the relationship between impaired gait adaptability and fall risk in older people. Gait Posture. 2018; 59:188–192. PMID:
29055270.

54. Volterrani M, Clark AL, Ludman PF, et al. Predictors of exercise capacity in chronic heart failure. Eur Heart J. 1994; 15:801–809. PMID:
8088269.

55. Hasegawa R, Islam MM, Lee SC, Koizumi D, Rogers ME, Takeshima N. Threshold of lower body muscular strength necessary to perform ADL independently in community-dwelling older adults. Clin Rehabil. 2008; 22:902–910. PMID:
18955422.

56. Kamiya K, Mezzani A, Hotta K, et al. Quadriceps isometric strength as a predictor of exercise capacity in coronary artery disease patients. Eur J Prev Cardiol. 2014; 21:1285–1291. PMID:
23723330.

57. Kamiya K, Masuda T, Tanaka S, et al. Quadriceps strength as a predictor of mortality in coronary artery disease. Am J Med. 2015; 128:1212–1219. PMID:
26169888.

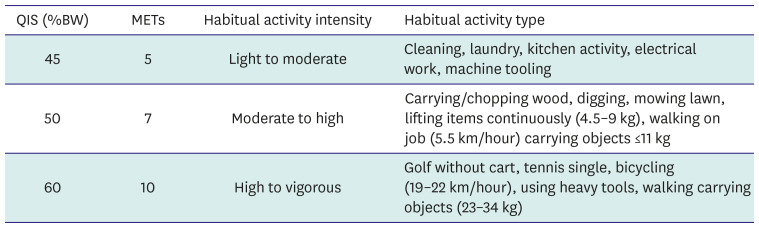
58. Ainsworth BE, Haskell WL, Herrmann SD, et al. 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011; 43:1575–1581. PMID:
21681120.
59. Nakamura T, Kamiya K, Hamazaki N, et al. Quadriceps strength and mortality in older patients with heart failure. Can J Cardiol. 2021; 37:476–483. PMID:
32622879.

60. Leon AS, Franklin BA, Costa F, et al. Cardiac rehabilitation and secondary prevention of coronary heart disease: an American Heart Association scientific statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Cardiac Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity), in collaboration with the American association of Cardiovascular and Pulmonary Rehabilitation. Circulation. 2005; 111:369–376. PMID:
15668354.

61. McAlister FA, Stewart S, Ferrua S, McMurray JJ. Multidisciplinary strategies for the management of heart failure patients at high risk for admission: a systematic review of randomized trials. J Am Coll Cardiol. 2004; 44:810–819. PMID:
15312864.

62. Davidson PM, Cockburn J, Newton PJ, et al. Can a heart failure-specific cardiac rehabilitation program decrease hospitalizations and improve outcomes in high-risk patients? Eur J Cardiovasc Prev Rehabil. 2010; 17:393–402. PMID:
20498608.

63. Ambrosetti M, Abreu A, Corrà U, et al. Secondary prevention through comprehensive cardiovascular rehabilitation: from knowledge to implementation. 2020 update. A position paper from the Secondary Prevention and Rehabilitation Section of the European Association of Preventive Cardiology. Eur J Prev Cardiol. 2021; 28:460–495.

64. Borg GA, Noble BJ. Perceived exertion. Exerc Sport Sci Rev. 1974; 2:131–153. PMID:
4466663.

65. Fletcher GF, Ades PA, Kligfield P, et al. Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation. 2013; 128:873–934. PMID:
23877260.
66. Piepoli MF, Conraads V, Corrà U, et al. Exercise training in heart failure: from theory to practice. A consensus document of the Heart Failure Association and the European Association for Cardiovascular Prevention and Rehabilitation. Eur J Heart Fail. 2011; 13:347–357. PMID:
21436360.

67. Haykowsky MJ, Daniel KM, Bhella PS, Sarma S, Kitzman DW. Heart Failure: Exercise-Based Cardiac Rehabilitation: Who, When, and How Intense? Can J Cardiol. 2016; 32:S382–S387. PMID:
27692119.

68. Kamiya K, Sato Y, Takahashi T, et al. Multidisciplinary cardiac rehabilitation and long-term prognosis in patients with heart failure. Circ Heart Fail. 2020; 13:e006798. PMID:
32986957.

69. Kamiya K, Yamamoto T, Tsuchihashi-Makaya M, et al. Nationwide survey of multidisciplinary care and cardiac rehabilitation for patients with heart failure in Japan - an analysis of the AMED-CHF study. Circ J. 2019; 83:1546–1552. PMID:
31189753.

70. Piña IL, Bittner V, Clare RM, et al. Effects of exercise training on outcomes in women with heart failure: analysis of HF-ACTION (Heart Failure-A Controlled Trial Investigating Outcomes of Exercise TraiNing) by sex. JACC Heart Fail. 2014; 2:180–186. PMID:
24720927.
71. Witvrouwen I, Van Craenenbroeck EM, Abreu A, Moholdt T, Kränkel N. Exercise training in women with cardiovascular disease: Differential response and barriers - review and perspective. Eur J Prev Cardiol. 2019; 28:779–790.

72. Rinaldo L, Caligari M, Acquati C, et al. Functional capacity assessment and minimal clinically important difference in post-acute cardiac patients: the role of Short Physical Performance Battery. Eur J Prev Cardiol. 2022; 29:1008–1014. PMID:
33846721.

73. Spertus J, Peterson E, Conard MW, et al. Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J. 2005; 150:707–715. PMID:
16209970.

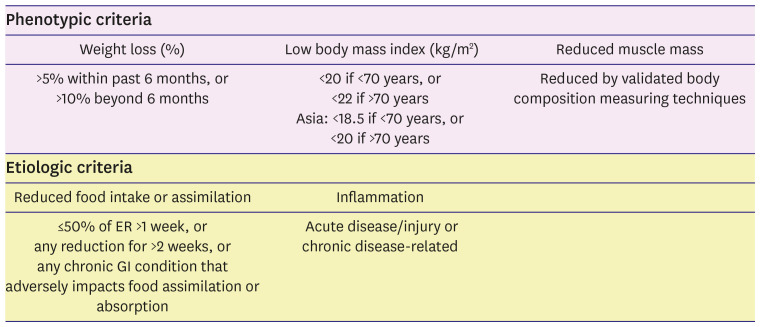
74. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001; 56:M146–M156. PMID:
11253156.

75. Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012; 60:1487–1492. PMID:
22881367.

76. Mudge AM, Denaro CP, Scott AC, et al. Addition of supervised exercise training to a post-hospital disease management program for patients recently hospitalized with acute heart failure: the EJECTION-HF randomized phase 4 trial. JACC Heart Fail. 2018; 6:143–152. PMID:
29413370.

77. Cooper LB, Mentz RJ, Sun JL, et al. Psychosocial factors, exercise adherence, and outcomes in heart failure patients: insights from Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION). Circ Heart Fail. 2015; 8:1044–1051. PMID:
26578668.

78. American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription. 9th ed. Philadelphia (PA): Lippincott Williams & Wilkins;2013.
79. Hodgson CL, Berney S, Harrold M, Saxena M, Bellomo R. Clinical review: early patient mobilization in the ICU. Crit Care. 2013; 17:207. PMID:
23672747.

80. Hermans G, De Jonghe B, Bruyninckx F, Van den Berghe G. Clinical review: critical illness polyneuropathy and myopathy. Crit Care. 2008; 12:238. PMID:
19040777.

81. Puthucheary ZA, Rawal J, McPhail M, et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013; 310:1591–1600. PMID:
24108501.

82. Yamashita M, Kamiya K, Matsunaga A, et al. Features of trunk muscle wasting during acute care and physical function recovery with aortic disease. J Cachexia Sarcopenia Muscle. 2022; 13:1054–1063. PMID:
35178890.

83. Topp R, Ditmyer M, King K, Doherty K, Hornyak J 3rd. The effect of bed rest and potential of prehabilitation on patients in the intensive care unit. AACN Clin Issues. 2002; 13:263–276. PMID:
12011598.

84. Bloomfield SA. Changes in musculoskeletal structure and function with prolonged bed rest. Med Sci Sports Exerc. 1997; 29:197–206. PMID:
9044223.

85. Kaneko H, Itoh H, Kamiya K, et al. Acute-phase initiation of cardiac rehabilitation and clinical outcomes in hospitalized patients for acute heart failure. Int J Cardiol. 2021; 340:36–41. PMID:
34454966.

86. Ueno K, Kamiya K, Kaneko H, et al. Acute-phase initiation of cardiac rehabilitation for short-term improvement in activities of daily living in patients hospitalized for acute heart failure. J Cardiovasc Dev Dis. 2022; 9:97. PMID:
35448073.

87. Kayambu G, Boots R, Paratz J. Early physical rehabilitation in intensive care patients with sepsis syndromes: a pilot randomised controlled trial. Intensive Care Med. 2015; 41:865–874. PMID:
25851383.

88. Liu N, Cadilhac DA, Andrew NE, et al. Randomized controlled trial of early rehabilitation after intracerebral hemorrhage stroke: difference in outcomes within 6 months of stroke. Stroke. 2014; 45:3502–3507. PMID:
25336514.

89. Zang K, Chen B, Wang M, et al. The effect of early mobilization in critically ill patients: a meta-analysis. Nurs Crit Care. 2020; 25:360–367. PMID:
31219229.

90. Goldfarb M, Semsar-Kazerooni K, Morais JA, Dima D. Early mobilization in older adults with acute cardiovascular disease. Age Ageing. 2021; 50:1166–1172. PMID:
33247593.

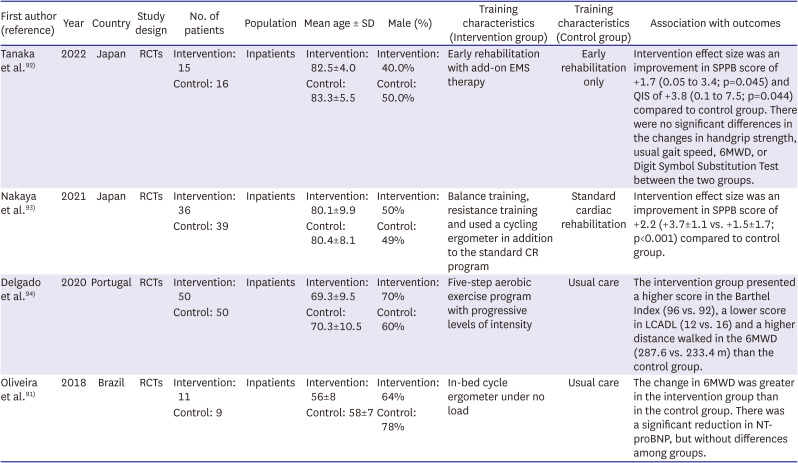
91. Oliveira MF, Santos RC, Artz SA, et al. Safety and efficacy of aerobic exercise training associated to non-invasive ventilation in patients with acute heart failure. Arq Bras Cardiol. 2018; 110:467–475. PMID:
29538506.
92. Tanaka S, Kamiya K, Matsue Y, et al. Effects of electrical muscle stimulation on physical function in frail older patients with acute heart failure: a randomized controlled trial. Eur J Prev Cardiol. 2022; 29:e286–e288. PMID:
35137047.

93. Nakaya Y, Akamatsu M, Ogimoto A, Kitaoka H. Early cardiac rehabilitation for acute decompensated heart failure safely improves physical function (PEARL study): a randomized controlled trial. Eur J Phys Rehabil Med. 2021; 57:985–993. PMID:
34291626.

94. Delgado BM, Lopes I, Gomes B, Novo A. Early rehabilitation in cardiology - heart failure: The ERIC-HF protocol, a novel intervention to decompensated heart failure patients rehabilitation. Eur J Cardiovasc Nurs. 2020; 19:592–599. PMID:
32316758.

95. Forestieri P, Guizilini S, Peres M, et al. A cycle ergometer exercise program improves exercise capacity and inspiratory muscle function in hospitalized patients awaiting heart transplantation: a pilot study. Rev Bras Cir Cardiovasc. 2016; 31:389–395.
96. Matsue Y, Kamiya K, Saito H, et al. Prevalence and prognostic impact of the coexistence of multiple frailty domains in elderly patients with heart failure: the FRAGILE-HF cohort study. Eur J Heart Fail. 2020; 22:2112–2119. PMID:
32500539.

97. Kortebein P, Ferrando A, Lombeida J, Wolfe R, Evans WJ. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA. 2007; 297:1772–1774. PMID:
17456818.

98. Groehs RV, Antunes-Correa LM, Nobre TS, et al. Muscle electrical stimulation improves neurovascular control and exercise tolerance in hospitalised advanced heart failure patients. Eur J Prev Cardiol. 2016; 23:1599–1608. PMID:
27271264.

99. Forestieri P, Bolzan DW, Santos VB, et al. Neuromuscular electrical stimulation improves exercise tolerance in patients with advanced heart failure on continuous intravenous inotropic support use-randomized controlled trial. Clin Rehabil. 2018; 32:66–74. PMID:
28633534.

100. Hirose S, Matsue Y, Kamiya K, et al. Prevalence and prognostic implications of malnutrition as defined by GLIM criteria in elderly patients with heart failure. Clin Nutr. 2021; 40:4334–4340. PMID:
33551220.

101. Cederholm T, Jensen GL, Correia MITD, et al. GLIM criteria for the diagnosis of malnutrition - a consensus report from the global clinical nutrition community. J Cachexia Sarcopenia Muscle. 2019; 10:207–217. PMID:
30920778.

102. Kootaka Y, Kamiya K, Hamazaki N, et al. The GLIM criteria for defining malnutrition can predict physical function and prognosis in patients with cardiovascular disease. Clin Nutr. 2021; 40:146–152. PMID:
32571679.

103. Heyland DK, Stapleton RD, Mourtzakis M, et al. Combining nutrition and exercise to optimize survival and recovery from critical illness: conceptual and methodological issues. Clin Nutr. 2016; 35:1196–1206. PMID:
26212171.

104. Mentz RJ, Whellan DJ, Reeves GR, et al. Rehabilitation intervention in older patients with acute heart failure with preserved versus reduced ejection fraction. JACC Heart Fail. 2021; 9:747–757. PMID:
34246602.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download