INTRODUCTION
During catheter ablation for atrial fibrillation (AF), non-pulmonary vein (non-PV) atrial arrhythmogenic activity is detected in a significant proportion of patients.
1) These non-PV foci that were confirmed to initiate AF have been reported in up to 11% of previous cohorts during AF ablation, and the prevalence of nonsustained atrial arrhythmias, including atrial premature beats, which are defined as non-PV triggers, has been reported to be up to 60%.
2) The major anatomical regions wherein the non-PV foci are clustered are the inferior mitral annulus, the posterior left atrial wall, the interatrial septum, the crista terminalis and the Eustachian ridge, the coronary sinus (CS), and the superior vena cava. Relatively rarely the left atrial appendage or the left superior vena cava (and its remnant—the ligament of Marshall) is reported to have arrhythmogenic activity.
3)4)
In 2010, Yamada et al.
5) first reported atrial tachycardia (AT) initiating AF that was successfully ablated in the noncoronary cusp (NCC) of the aorta. Their case report demonstrated the clinical AT from NCC that initiated AF, and the tailored approach targeting NCC AT alone completely eliminated the AF. Therefore, performing AT mapping in the NCC during AF procedure appears to be reasonable, if the local His-bundle area atrial activation of clinical AT precedes P-wave. However, the prevalence and characteristics of non-PV that triggers activity from the NCC in AF ablation procedures are not yet known.
For reference, the distinctive electrophysiological characteristics of de novo NCC AT without AF are described infrequently. According to previous studies, AT ablation at NCC was performed in 4.1–8.8% of all patients with AT.
6)7)8) The P-wave morphology was predominantly negative in the inferior leads and biphasic in leads V1 and V2.
8) Sometimes, varying P-wave morphology may be a clinical clue indicating that the site of the AT origin is intermediate between the 2 atria and suggests that the AT originates from the NCC.
9)
In this study, our aim was to investigate the prevalence and clinical characteristics of NCC AT uncovered during an AF ablation procedure, which was compared with de novo NCC AT in patients without AF.
METHODS
Ethical statement
This study was approved by the Institutional Review Board (IRB) of each participating center, the need for informed consent was also waived by IRB. The IRB number of the main center is S2020-2924-0003 (Asan Medical Center).
Study population
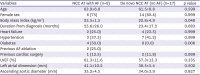
From the establishment year of the cardiac electrophysiology laboratory in each center to December 2020, a total of 10,178 patients who underwent radiofrequency catheter ablation for AF were screened in 11 tertiary hospitals, and their medical records were retrospectively reviewed. All patients had symptomatic paroxysmal or persistent AF and documented failure of or intolerance to at least one antiarrhythmic drug.
Study protocol
The AT ablation at the NCC during AF ablation procedure was identified for analysis from the AF registry. For its comparator, de novo NCC AT ablation cases without AF history or inducible AF during procedure were identified from the AT ablation registries in the same 11 hospitals. The prevalence and clinical and electrophysiological characteristics of NCC AT with AF among all AF ablation cases and de novo NCC AT without AF were described and compared.
Electrophysiological mapping and ablation
All patients with AF underwent antral PV isolation (PVI) guided by the electroanatomical mapping system. Electroanatomical mapping was performed using the CARTO system (Biosense Webster, Diamond Bar, CA, USA) or the EnSite NAVX Navigation system (St. Jude Medical, Inc, St. Paul, MN, USA), depending on each institution. Every procedure protocol ablation setting and the choice of catheters determined by each operator was included for the study analysis. Cases with additional substrate-based ablations were also included.
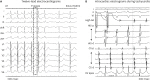
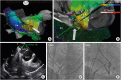
The provocation protocol to elicit non-PV triggers was different; therefore, all operators’ decision of individual protocol for evaluating non-PV triggers was respected. In all the designated hospitals, the stimulation protocol consisted of programmed stimulation at 2 basic cycle lengths with up to 2 extrastimuli and burst pacing at the right atrium, left atrium (LA), or right ventricular (RV) apex. If required, intravenous isoproterenol was administered to provoke the tachycardia. After the initiation of tachycardia, the diagnosis of focal AT was confirmed by the differential pacing maneuver to distinguish it from atrioventricular reentrant or nodal reentrant tachycardia. If AT was induced after PV isolation, the optimal electrophysiology study was evaluated to confirm AT. The origin of AT inside the NCC was confirmed by either aortography or intracardiac echocardiography. To determine P-wave morphology during tachycardia, procedural data were thoroughly reviewed, and found the P-waves not overlapped with T-waves
Statistical analysis
We described the prevalence and electrocardiographic and electrophysiolgical characteristics of the NCC AT cases, complications, and clinical outcomes. Categorical data were expressed as absolute frequencies and percentages where appropriate. p values were determined by Mann-Whitney U test or Fisher’s exact test. Parametric data were presented using descriptive statistics (mean±SD). The threshold of significance was set at a 2-sided p value of <0.05. Statistical analyses were performed using the Statistical Package for the Social Sciences for Windows (SPSS version 21.0; IBM Corp, Armonk, NY, USA).
DISCUSSION
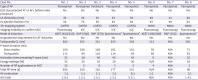
Based on this multicenter retrospective registry study, we reported the epidemiology and clinical characteristics of the uncommon ATs from the NCC (0.08%) uncovered during AF catheter ablation. To the best of our knowledge, this study is the first and largest to describe the detailed characteristics of NCC ATs with AF compared with de novo NCC ATs. Our new observations are as follows: (1) NCC ATs uncovered after PVI were rare, with a prevalence of overall 0.08% (0.07% in initial AF ablation cases, and 0.15% in redo cases), (2) The tachycardia cycle length was significantly shorter than that of de-novo NCC ATs, and presented mostly 1:1 conduction, (3) Compared to de-novo NCC ATs, the P-wave duration changes from sinus rhythm to AT was significantly higher in NCC ATs with AF, because baseline P-wave duration is prolonged in AF cases. (4) The AF recurrence rate (1 of 8, 12.5%) after PVI and NCC AT ablation was low without significant procedure related complication
Therefore, AT from the NCC should be suspected if unusual AT induced during AF ablation exhibits broad anteroseptal propagation with a 1:1 AV conduction and earliest atrial electrogram at the proximal coronary sinus (CS) catheter. This type of AT should be differentiated from other supraventricular tachycardias (SVTs) such as macroreentry (cavotricuspid isthmus or mitral isthmus-dependent flutter), AT from the interatrial septum or inside CS, atrioventricular reentrant tachycardia using the septal bypass tract, and atrioventricular nodal reentrant tachycardia. The mechanisms and site of the origin of SVT can be defined and classified by analyzing the specific phenomena of NCC AT, including the following: (1) the mode of initiation of the tachycardia is generally spontaneous or by the atrial pacing protocol without the need of isoproterenol, (2) the atrial activation sequence displays anteroseptal propagation (P-wave differs from sinus) and the relationship of P-wave with the QRS complex generally shows 1:1 conduction (transient AV block may exist without affecting SVT), (3) macroreentrant tachycardia might be excluded through electrophysiology study, (4) overdrive pacing from the proximal/distal CS, cavotricuspid isthmus, or RV apex could be helpful to specify the site of origin and to rule out reentrant SVT, (5) the response to atrial and/or ventricular extrastimulation during tachycardia is useful to explore the role of atrial, His-bundle, or ventricular participation in the tachycardia and can be used to distinguish AT.
The localization of nonsustained non-PV triggers remains challenging.
10) The P-wave morphology and the intra-atrial multipolar activation pattern and timing can help identify non-PV trigger sites of origin. Kubala et al.
11) described the electrophysiological characteristics of 15 major sites of non-PV triggers, but they did not include the NCC in their study. Therefore, we should speculate the characteristics of non-PV triggers from the NCC based on the unique characteristics of focal AT from the NCC. The electrophysiological characteristics of focal AT near the aortic cusps have been previously reported.
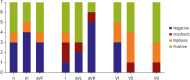
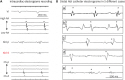
12) First, the P-wave duration is shorter than that during the sinus rhythm in the usual interatrial septal non-PV triggers like septal PACs. In the present study, we have reported the same observation. Second, the P-wave morphology is an important discriminative feature of this type of AT focus. P-waves are positive in leads I and augmented vector left (aVL).
5) However, in our study, the P-wave morphology was variable, and only 50% of lead I and 63% of lead aVL exhibited positive P-waves. In other previous research, the P-waves of NCC AT demonstrated negativity in the inferior leads and a biphasic pattern in the right precordial leads.
13) In our study, the de novo ATs also presented a similar morphological trend (
Supplementary Figure 9). However, in the 8 study cases with AF, 50–63% of P-waves in the inferior leads were negative, and only 25–38% of P-waves in the right precordial leads were biphasic. Moreover, as the cavotricuspid isthmus-dependent flutter that occurs after the LA ablation of AF has atypical ECG characteristics because of altered LA activation, the ECG findings could be different between ne novo NCC AT and NCC AT after PVI.
14) Hence, NCC ATs should not be ruled out with P-wave morphologies and must be suspected in anteroseptal propagating ATs irrespective of P-wave morphologies.
Studies have reported that the NCC region is an important origin of focal AT from the aortic root.
8)15) However, the anatomical substrate responsible for atrial arrhythmias in the NCC is unknown. Based on the autopsy data of human hearts, Gami et al.
16) reported that the NCC region was immediately adjacent to and contiguous with the interatrial septum in 603 of 603 (100%) hearts. It is unclear whether the atrial myocardium in the NCC is a source of arrhythmias or whether a catheter tip inside the NCC preoccupies an advantageous position to treat atrial arrhythmias in adjacent atrial areas.
17) Anatomically, the aortic root is also adjacent to the epicardial atrial myocardium and occupies a central location within the tricuspid and mitral annulus in a normal heart. Especially, the rightward margin of the NCC is related to the paraseptal region of the right atrial wall, whereas the leftward margin is related to the left atrial wall.
6) Moreover, the aortic root is adjacent to the His-bundle area. Several studies have suggested that para-Hisian ATs can be successfully ablated in the NCC in the aorta.
12)18) Therefore, further investigation is required on the characteristics of AT that is successfully ablated in the NCC and its role as a non-PV trigger.
Clinically, the AT from aortic cusps generally exhibits abrupt onset and offset of the tachycardia and easy induction and termination via atrial stimulation.
13) We also observed that the NCC ATs were easily induced spontaneously or by atrial stimulation. No patient required isoproterenol for inducing ATs. Unlike the transient or rapidly degenerating nature of alleged non-PV triggers,
1) the NCC ATs examined in the present study demonstrated easy inducibility without isoproterenol, ability to sustain, and rapid conduction property to the ventricle. We speculate that the NCC AT uncovered after PVI is not influenced by the stimulation techniques or the provocative maneuver, but further studies on this type of AT are necessary. This study may provide useful clinical information for determining a successful ablation site at the NCC. The local atrial electrogram at a successful ablation site generally has greater amplitude than that of the ventricular electrogram.
8) Although the power or total number of RF energy applications varied among cases, all treatments were successful in eliminating the clinical AT.
The tachycardia cycle length of 8 study cases with AT combined with AF was significantly shorter than that of the de novo NCC ATs. This may be because of the vagal denervation after PVI. Circumferential PVI often causes vagal reflexes during the procedure due to the coincidental modification of ganglionated plexus, which is located on the PV antrum.
19) Resting sinus heart rate is known to increase after PVI,
20) and the heart rate of focal ATs might be changed after the modification of cardiac autonomic innervation by PVI. However, it is difficult to determine whether the tachycardia cycle length of AT has changed due to PVI or whether the faster ATs tend to easily trigger AF than the slower ATs.
The potential risk of catheter ablation near the His-bundle area includes heart block.
21) Moreover, complete AV block was reported in AT ablation inside the RCC.
6) In contrast, in those types of AT cases, catheter ablation within the base of the NCC represented a safe and effective procedure outcome in the previous reports. In the recent study, Yamabe et al.
12) reported that ablation of AT arising near the atrioventricular node from NCC is effective and safe. Ouyang et al.
6) reported successful catheter ablation in the NCC for focal AT near the His-bundle region without AV block in 9 patients. Wang et al.
22) recommended the NCC as a preferential ablation site for focal ATs surrounding the anterior atrial septum. In our cases, there were no peri- or post-procedural AV block events. Therefore, mapping and ablation at the NCC should be considered when a non-PV trigger has a specific electrophysiological morphology indicating the location of the His-bundle region.
Although this study has the largest series of patients with NCC AT with or without AF catheter ablation, it is limited by its retrospective nature. Although we included experienced tertiary AF centers, the non-standardized AF ablation procedure and the non-PV trigger provocation protocol, which does not certify careful inspection inside the NCC, may lead to underestimation of the prevalence of NCC triggers. Second, this study does not ensure that NCC ATs in the current study actually play a role as non-PV triggers, because all ATs were uncovered after PVI. Further research is needed on the characteristics of NCC ATs, which are clinically important in triggering AF. Third, the P-wave morphologies and local ECG analyses were performed as its previously recorded form during the index procedure although they were re-evaluated by expert cardiac electrophysiologists. Ventricular rapid pacing might aid in uncovering the clear morphologies of P-waves during AT with 1:1 conduction. The accurate differentiation between 12-lead ECG and local electrogram at ablation site would be required because of possibility of passive activation. Fourth, the number of analyzed cases is too small to define AT characteristics. The statistical power was too weak to compare the 2 AT groups with or without AF in detail due to the small number of cases. However, it would be better to use our study results as a reference for future studies, and not as conclusive data. Lastly, supporting data such as aortograms was not available in all presented cases. Although all cases were performed by expert electrophysiologists, the measurement or observations might be different in detail due to physician preference.
In conclusion, the prevalence of rare NCC ATs newly uncovered during AF ablation was 0.08%. The ATs propagating from the anteroseptal area should be mapped inside the NCC, irrespective of P-wave characteristics. The tachycardia rate was higher in NCC ATs with AF than that of de novo NCC ATs, although there was no difference in baseline characteristics. The success rate of NCC AT was high and the AF recurrence rate after both PVI and NCC AT ablation was low without significant procedure related complication such as AV block.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download