Abstract
Pituitary surgery has advanced considerably in recent years with the exploration and development of various endoscopic approaches and techniques. Different endoscopic skull base approaches are being applied to access sellar tumors in different locations. Moreover, extracapsular dissection and cavernous sinus exploration have enabled gross total resection of sellar tumors where it could not have been achieved in the past. Techniques for skull base reconstruction have also progressed, allowing surgeons to remove larger and more complicated tumors than before. This review article discusses different endoscopic skull base approaches, surgical techniques for removing pituitary adenomas, and reconstruction methods for repairing postoperative low-flow and high-flow cerebrospinal fluid leakage.
Pituitary adenomas are the second most common class of brain tumors, with a prevalence of 80–100 cases per 100,000 people [1,2]. Classically, microscopic transsphenoidal surgery was performed to remove these tumors. However, the deep location of these tumors and the narrow surgical space and window created by the retractors resulted in poor visualization, which posed a major obstacle. Advances in optics and technical progress in electric displays have brought endoscopy into transnasal brain surgery. Endoscopy has improved surgical visualization of tumors and the surrounding normal tissue by providing a panoramic view. Ultimately, the widening of the surgical window has led to an improvement in the tumor resection rate compared to the microscopic approach [3,4].
Over the decades, the documented anatomical knowledge and surgical experiences have expanded the indications for endoscopic endonasal surgery and permitted approaches to parasellar areas, such as the planum/tuberculum sellae, cavernous sinus (CS), and clivus, where larger and more complicated pituitary adenomas invade. On the other hand, more extensive surgical approaches have led to larger skull base defects and higher intraoperative cerebrospinal fluid (CSF) leakage rates. Thus, robust skull defect reconstruction techniques became essential to prevent these postoperative complications, such as CSF leakage, meningitis, and pneumocephalus. In this review article, we discuss various advanced endoscopic skull base approaches and surgical techniques for pituitary adenomas. This current study was approved by the Institutional Review Board of Seoul National University Hospital (No. 2206-015-1329), and the requirement for informed consent was waived owing to the retrospective nature of the study.
A great majority of pituitary adenomas can be removed via the transsphenoidal transsellar route. To visualize the whole sella, a wide sphenoidotomy is required because the sella is usually enlarged anteriorly, laterally and inferiorly by the tumor. The sellar bone is removed up to the four blue lines corresponding to the bilateral CS and the superior and inferior intercavernous sinuses. Some sellar tumors extending laterally to the CS can also be removed using this approach [5].
The pseudocapsule of a pituitary adenoma is a lining of normal pituitary gland cells formed due to gradual compression of the gland by the tumor [6]. Before this pseudocapsule was explored as a viable surgical plane, resection of pituitary adenomas was performed using intracapsular dissection. The pseudocapsule was immediately opened along with the dura, and the tumor was removed in a piecemeal fashion using ring curettes. However, this form of resection relied solely on the surgeon’s ability to differentiate the tumor from the normal pituitary gland by recognizing the texture, color and consistency of the tumor [7]. Problems with leaving a large amount of residual tumor behind or inadvertently damaging the normal gland structure were reported, as even the most experienced surgeons sometimes had difficulty differentiating the tumor from the normal gland.
To overcome the above problems, surgeons started to implement extracapsular dissection. After a wide visualization of the sella, the dura was opened carefully while maintaining the integrity of the compressed paper-thin pituitary gland and its pseudocapsule. A plane between the pituitary gland and pseudocapsule was dissected and followed in order to achieve extracapsular dissection of the tumor. Instead of removing the tumor in a piecemeal fashion, surgeons were thus able to “peel off” the tumor from the compressed pituitary gland or the dura (Fig. 1). Since this dissection was performed under direct endoscopic visualization of the tumor and its periphery, extracapsular dissection had advantages over the intracapsular approach. Most importantly, the tumor could be reliably distinguished from the surrounding structure. Furthermore, this technique allowed the exact site of CS invasion, if any, to be identified. This allowed surgeons to achieve complete removal of the tumor with confidence [8]. Theoretically, due to its aggressive nature, extracapsular dissection may increase the risk of surgical damage to the surrounding structure. However, direct visualization of these critical structures seems to offset such risks. To date, numerous studies have reported excellent surgical results for both nonfunctioning and functioning pituitary adenomas, with no significant increase in postoperative complications [9-12].
For both nonfunctioning and functioning pituitary adenomas, gross total resection is the most important prognostic factor for good tumor control and endocrinological cure [13]. However, large tumors, especially those with CS invasion, have often been considered as obstacles to achieving such results [14]. CSs are complex venous dural sinuses housing important neurovascular structures, including the internal carotid artery (ICA) and cranial nerves, making tumor resection in these spaces particularly challenging [15]. A CS is defined by four walls: medial, lateral, posterior and superior [16]. Resection of these walls, especially the medial CS wall, to achieve access to the CS has been explored over the years and has now been widely adopted. This technique has provided an effective and a safe way for improved visualization of the tumor, which has led to a greater degree of tumor removal and has significantly increased the chance of endocrinological remission [15,17-22].
Truong et al. [23] published a detailed article on surgical techniques for medial CS wall resection. They distinguished the sellar medial wall, which covers the pituitary gland, from the anterior wall, which corresponds to the sphenoidal part of the medial wall. The concept of medial CS wall removal proceeds from the fact that both the medial and the anterior wall are made of a single dural layer with distinct origins. The single-layered anterior wall can usually be directly exposed at the lowest aspect, which is considered the safest location. After the anterior wall is exposed, the inferior petrosal ligament anchoring the medial wall to the anterior CS wall can be cut to mobilize the medial wall away from the ICA. A subsequent cut through the sellar floor and the sellar roof is then performed. Finally, the caroticoclinoid ligament (CCL), which anchors the medial CS wall superiorly, can be cut, releasing the medial CS wall (Fig. 2A, B). An alternative method of entering the CS can be achieved by dissecting the inner and outer dura of inferior intercavernous sinus (Fig. 2C, D) [24], which make up the medial and anterior CS walls, respectively. This allows access to the CS without violating the anterior wall of CS overlying the ICA.
Furthermore, endoscopically accessing the clinoidal venous space dorsal to the ICA, known as the dorsal clinoidal space (DCS), was recently explored [25]. The DCS is formed by a meningeal dural layer, a distal dural ring and a CCL, which make up its medial wall, roof, and floor, respectively. Previously, the DCS was not accessible during transcranial surgeries due to obstruction by the ICA and was a potential site for residual tumors when the distal dural ring was invaded. The DCS can be accessed by first extensively exposing the sellar and parasellar areas. The sellar dura is opened and extended inferolaterally to separate the anterior and medial walls of the CS. Along this plane, the ICA is identified and dissected away from the medial wall of the CS up to the level of the CCL. A blunt dissector is then used to dissect the space below and above the CCL and finally the medial CS wall from the clinoidal ICA to gain access to DCS. Understanding the anatomy of DCS and physically being able to access it have increased the chances of gross total resection of the tumor.
Similar to tumors with CS invasion, tumors with suprasellar extension into the third ventricle have historically posed great challenges due to their deep location and the critical nature of the surrounding structures [26]. An extended endoscopic transsphenoidal approach has now been widely adopted and has been demonstrated in various studies to be an effective method of removing third ventricle tumors [27-30]. Seo et al. [31] described the surgical methods and outcomes of 82 patients with these types of tumors. After the appropriate portion of the skull base was drilled out and the dura was opened, arachnoid dissection was first performed to protect the anterior communicating artery and/or superior hypophyseal artery. The tumor was dissected gently under direct visualization, and one of the two surgical corridors was chosen according to the characteristics of the tumor to gain access to the third ventricle: a suprachiasmatic approach through the lamina terminalis or an infrachiasmatic approach through the tuber cinereum (Fig. 3). The tumor capsule was preferentially dissected in an en bloc fashion following the glial plane to avoid injury to the pia mater. The surgeon carefully dissected the tumor while minimizing injury to the lateral wall of the third ventricle and the hypothalamus. Gross total resection was achieved in 86.5% of the patients, with a visual improvement rate of 81.6%.
An appropriate endoscopic approach should be chosen to gain optimal surgical access to pituitary tumors with extensive growth. The extent of skull base exposure should be tailored to the extent of the tumor. This applies to pituitary adenomas that do not have significant superior extension but extend anteriorly to the frontal base, laterally beyond the lateral border of the ICA or posteriorly down to the clivus.
The endoscopic transtubercular/transplanar approach is an anterior and superior extension of the transsellar approach where additional tuberculum sellae and planum sphenoidale are removed after opening the sellar floor (Fig. 4). The degree of rostral opening is dependent on the extent of the tumor, whereas the degree of lateral opening is limited by the medial opticocarotid recess [32]. This approach provides excellent access to tumors with major frontal and/or suprasellar extension [33], which otherwise would have required a transcranial approach. These include pituitary adenomas with anterior cerebral artery (ACA) adhesion and sellar tumors invading the third ventricle. When the outer arachnoid membrane is used as a dissection plane, the sellar and frontal extensions of the tumor can be removed under direct visualization while protecting the frontal cortex and the ACA. This technique does not involve excessive brain traction as seen in the transcranial approach; therefore, less damage is inflicted on the diencephalon and the optic nerve area [34]. However, a transcranial approach may be considered when a cuff of the frontal lobe is present between the anterior extent of the tumor and the skull base (Fig. 5).
The endoscopic transclival approach is appropriate for accessing midline clival tumors or brainstem lesions. It can provide a whole range of access to tumors of the upper clivus with dorsum sellae extension to the lower clivus [35]. However, it is limited in its ability to provide access to lateral areas [36], and it is limited by the ICAs superiorly and by the hypoglossal and jugular foramens inferiorly (Fig. 6) [37].
The endoscopic trans-pterygoid palatine fossa approach can be used to access tumors extending into the lateral recess of the sphenoid sinus, as well as invasive sellar tumors (e.g., pituitary macroadenomas) involving the lateral portion of the CS (Fig. 7). The surgical approach starts with removal of the middle turbinate, uncinate process, and bulla ethmoidalis. The sphenoid sinus is opened, and maxillary antrostomy is performed to expose the posterior wall of the maxillary sinus, which is the face of the pterygoid palatine fossa. After the posterior wall is removed, the sphenopalatine artery is cauterized, and the pterygopalatine fossa is opened. The Vidian nerve, foramen rotundum, and maxillary nerve are identified and retracted laterally, allowing continuation of the bone work to ultimately gain access to the CS [38-41]. The advantage of this approach is that the entire lateral sphenoid and CS can be directly visualized. However, to utilize this approach, the tumor must be large enough that the space around the tumor allows sufficient surgical working space within the sinus [35]. Therefore, tumors appropriate for this approach usually have an increased chance of laterally displacing the ICA due to their size, leading to an increased chance of ICA morbidity as the surgeon approaches the tumor.
CSF leaks can mainly be divided into “low-flow” and “high-flow” CSF leaks. A low-flow CSF leak is defined as small drops of CSF leakage occurring during transient increases in intracranial pressure, for example, while the patient is coughing or straining. Most CSF leaks after endoscopic removal of pituitary adenoma, if present, fall within this category. In contrast, a high-flow CSF leak refers to constant CSF flow caused by direct opening of the ventricles or connection with the cisterns [42,43]. It usually occurs following an extended transsphenoidal approach or in cases of revision surgery of the recurred pituitary adenoma. Various reconstruction techniques to repair these CSF leaks using different autologous (fat, fascia, cartilage, bone), allogenic and artificial materials (fibrin sealants) have been explored [44-46]. Although there is no consensus on what grafts and materials to use, a “multilayered” reconstruction method is important, especially for high-flow CSF leaks.
Low-flow CSF leaks can usually be repaired by sealing the defect with fibrin sealants, spraying tissue glue on top and positioning a temporary soft buttress in the nostrils to hold the repair construct in position. Some groups prefer to use autologous fat grafts to fill the sellar dead space [45,47] before sealing the defect with fibrin sealants; however, this does not seem necessary in most cases. Reconstruction for high-flow CSF leaks usually requires more layers to provide a more secure repair construct. Numerous studies have emphasized the importance of using pedicled vascularized flaps to repair high-flow CSF leaks [48]. A vascularized flap decreases the risk of postoperative CSF leakage, prevents infections at surgical sites, and promotes the healing process [49,50]. Some centers utilize lumbar drainage for CSF diversion as an adjunctive measure for repairing high-flow CSF leaks. However, recent studies have failed to show the benefits of lumbar drain placement in patients undergoing endoscopic skull base surgery [51,52]. Therefore, routine use of lumbar drains may be unnecessary even in high-flow CSF leaks if multilayered repair is achieved appropriately.
Some groups have explored questions regarding the necessity of rigid reconstruction. These groups have pointed out that vascularized flaps do not provide an immediate water-tight closure, thereby leaving high-risk patients vulnerable to CSF leakage until the flap is completely healed. Korean neurosurgeons have explored an innovative multilayered onlay reconstruction method in patients with intraoperative high-flow CSF leakage using a fibrin sealant patch, hydroxyapatite cement and a pedicled nasoseptal flap [53,54]. The bony defect was first sealed with a fibrin sealant patch to provide a temporary seal. Hydroxyapatite cement was then applied on top of the patch to provide a hard cover. After the cement was dried, it was covered with the nasoseptal flap. This method proved to be a reliable reconstruction method, with a postoperative CSF leakage rate of only 1.7%. It provided an immediate water-tight closure, allowing postoperative patients to ambulate freely without lumbar drainage. However, it was associated with an elevated risk of infection, warranting watchful follow-up.
There have been significant advances in pituitary surgery over recent years. Different endoscopic skull base approaches have been explored and are actively being applied to remove tumors that could not have been readily accessed in the past. Furthermore, extracapsular dissection and cavernous wall dissection have enabled surgeons to achieve gross total resection of sellar tumors under direct surgical visualization while minimizing damage to normal structures. As more thorough skull base reconstruction techniques develop, the application of endoscopy for removing sellar and parasellar tumors is likely to expand greatly in the future as surgeons continue to explore and push the boundaries of this field.
REFERENCES
1. Chanson P, Raverot G, Castinetti F, Cortet-Rudelli C, Galland F, Salenave S, et al. Management of clinically non-functioning pituitary adenoma. Ann Endocrinol (Paris). 2015; 76:239–47.

2. Molitch ME. Diagnosis and treatment of pituitary adenomas: a review. JAMA. 2017; 317:516–24.
3. Jho HD. Endoscopic transsphenoidal surgery. J Neurooncol. 2001; 54:187–95.
4. Frank G, Pasquini E, Farneti G, Mazzatenta D, Sciarretta V, Grasso V, et al. The endoscopic versus the traditional approach in pituitary surgery. Neuroendocrinology. 2006; 83:240–8.

5. Taniguchi M, Hosoda K, Akutsu N, Takahashi Y, Kohmura E. Endoscopic endonasal transsellar approach for laterally extended pituitary adenomas: volumetric analysis of cavernous sinus invasion. Pituitary. 2015; 18:518–24.

6. Qu X, Xu G, Qu Y, Song T. The pseudocapsule surrounding a pituitary adenoma and its clinical significance. J Neurooncol. 2011; 101:171–8.

7. Chamoun R, Takashima M, Yoshor D. Endoscopic extracapsular dissection for resection of pituitary macroadenomas: technical note. J Neurol Surg A Cent Eur Neurosurg. 2014; 75:48–52.

8. Oldfield EH, Vortmeyer AO. Development of a histological pseudocapsule and its use as a surgical capsule in the excision of pituitary tumors. J Neurosurg. 2006; 104:7–19.

9. Kinoshita Y, Tominaga A, Usui S, Arita K, Sakoguchi T, Sugiyama K, et al. The surgical side effects of pseudocapsular resection in nonfunctioning pituitary adenomas. World Neurosurg. 2016; 93:430–5.e1.

10. Jagannathan J, Smith R, DeVroom HL, Vortmeyer AO, Stratakis CA, Nieman LK, et al. Outcome of using the histological pseudocapsule as a surgical capsule in Cushing disease. J Neurosurg. 2009; 111:531–9.

11. Taylor DG, Jane JA, Oldfield EH. Resection of pituitary macroadenomas via the pseudocapsule along the posterior tumor margin: a cohort study and technical note. J Neurosurg. 2018; 128:422–8.

12. Zhang X, Wang YG, Tan J, Zhao G, Ma M, Chen J, et al. Comparison of outcomes between intracapsular resection and pseudocapsule-based extracapsular resection for pituitary adenoma: a systematic review and meta-analysis. BMC Neurol. 2022; 22:52.

13. Omar AT, Munoz DG, Goguen J, Lee JM, Rotondo F, Kovacs K, et al. Resection of the medial wall of the cavernous sinus in functioning pituitary adenomas: technical note and outcomes in a matched-cohort study. Clin Neurol Neurosurg. 2020; 106306.

14. Kim JH, Lee JH, Lee JH, Hong AR, Kim YJ, Kim YH. Endoscopic transsphenoidal surgery outcomes in 331 nonfunctioning pituitary adenoma cases after a single surgeon learning curve. World Neurosurg. 2018; 109:e409–16.

15. Dhandapani S, Singh H, Negm HM, Cohen S, Anand VK, Schwartz TH. Cavernous sinus invasion in pituitary adenomas: systematic review and pooled data meta-analysis of radiologic criteria and comparison of endoscopic and microscopic surgery. World Neurosurg. 2016; 96:36–46.

16. Rhoton AL Jr. The cavernous sinus, the cavernous venous plexus, and the carotid collar. Neurosurgery. 2002; 51(4 Suppl):S375–410.

17. Rutkowski M, Zada G. Management of pituitary adenomas invading the cavernous sinus. Neurosurg Clin N Am. 2019; 30:445–55.

18. Ferreli F, Turri-Zanoni M, Canevari FR, Battaglia P, Bignami M, Castelnuovo P, et al. Endoscopic endonasal management of non-functioning pituitary adenomas with cavernous sinus invasion: a 10-year experience. Rhinology. 2015; 53:308–16.
19. Cohen-Cohen S, Gardner PA, Alves-Belo JT, Truong HQ, Snyderman CH, Wang EW, et al. The medial wall of the cavernous sinus. Part 2: Selective medial wall resection in 50 pituitary adenoma patients. J Neurosurg. 2018; 131:131–40.

20. Nishioka H, Fukuhara N, Horiguchi K, Yamada S. Aggressive transsphenoidal resection of tumors invading the cavernous sinus in patients with acromegaly: predictive factors, strategies, and outcomes. J Neurosurg. 2014; 121:505–10.

21. Juraschka K, Khan OH, Godoy BL, Monsalves E, Kilian A, Krischek B, et al. Endoscopic endonasal transsphenoidal approach to large and giant pituitary adenomas: institutional experience and predictors of extent of resection. J Neurosurg. 2014; 121:75–83.

22. Park HH, Kim EH, Ku CR, Lee EJ, Kim SH. Outcomes of aggressive surgical resection in growth hormone-secreting pituitary adenomas with cavernous sinus invasion. World Neurosurg. 2018; 117:e280–9.

23. Truong HQ, Lieber S, Najera E, Alves-Belo JT, Gardner PA, Fernandez-Miranda JC. The medial wall of the cavernous sinus. Part 1: Surgical anatomy, ligaments, and surgical technique for its mobilization and/or resection. J Neurosurg. 2018; 131:122–30.

24. Nagata Y, Takeuchi K, Yamamoto T, Ishikawa T, Kawabata T, Shimoyama Y, et al. Removal of the medial wall of the cavernous sinus for functional pituitary adenomas: a technical report and pathologic significance. World Neurosurg. 2019; 126:53–8.

25. Xu Y, Nunez MA, Mohyeldin A, Vigo V, Mao Y, Cohen-Gadol AA, et al. Microsurgical anatomy of the dorsal clinoidal space: implications for endoscopic endonasal parasellar surgery. J Neurosurg. 2022; Feb. 4. [Epub]. https://doi.org/10.3171/2021.12.JNS211974.

26. Yasargil MG, Abdulrauf SI. Surgery of intraventricular tumors. Neurosurgery. 2008; 62(6 Suppl 3):1029–40.

27. Dho YS, Kim YH, Se YB, Han DH, Kim JH, Park CK, et al. Endoscopic endonasal approach for craniopharyngioma: the importance of the relationship between pituitary stalk and tumor. J Neurosurg. 2018; 129:611–9.

28. Cavallo LM, Di Somma A, de Notaris M, Prats-Galino A, Aydin S, Catapano G, et al. Extended endoscopic endonasal approach to the third ventricle: multimodal anatomical study with surgical implications. World Neurosurg. 2015; 84:267–78.

29. Cavallo LM, Prevedello DM, Solari D, Gardner PA, Esposito F, Snyderman CH, et al. Extended endoscopic endonasal transsphenoidal approach for residual or recurrent craniopharyngiomas. J Neurosurg. 2009; 111:578–89.

30. Phi JH, Kim YH, Kim JH, Kim DG. Clinical and anatomic features of supraglandular pituitary adenomas. World Neurosurg. 2016; 92:241–8.

31. Seo Y, Kim YH, Kim JH, Kong DS, Dho YS, Kang H, et al. Outcomes of the endoscopic endonasal approach for tumors in the third ventricle or invading the third ventricle. J Clin Neurosci. 2021; 90:302–10.

32. de Divitiis E, Cavallo LM, Cappabianca P, Esposito F. Extended endoscopic endonasal transsphenoidal approach for the removal of suprasellar tumors: Part 2. Neurosurgery. 2007; 60:46–58.
33. Barazi SA, Pasquini E, D’Urso PI, Zoli M, Mazzatenta D, Sciarretta V, et al. Extended endoscopic transplanum-transtuberculum approach for pituitary adenomas. Br J Neurosurg. 2013; 27:374–82.

34. Fomichev D, Kalinin P, Kutin M, Sharipov O. Extended transsphenoidal endoscopic endonasal surgery of suprasellar craniopharyngiomas. World Neurosurg. 2016; 94:181–7.

35. Kim YH, Jeon C, Se YB, Hong SD, Seol HJ, Lee JI, et al. Clinical outcomes of an endoscopic transclival and transpetrosal approach for primary skull base malignancies involving the clivus. J Neurosurg. 2018; 128:1454–62.

36. Karadag A, Senoglu M, Middlebrooks EH, Kinali B, Guvencer M, Icke C, et al. Endoscopic endonasal transclival approach to the ventral brainstem: radiologic, anatomic feasibility and nuances, surgical limitations and future directions. J Clin Neurosci. 2020; 73:264–79.

37. Essayed WI, Singh H, Lapadula G, Almodovar-Mercado GJ, Anand VK, Schwartz TH. Endoscopic endonasal approach to the ventral brainstem: anatomical feasibility and surgical limitations. J Neurosurg. 2017; 127:1139–46.

38. Fortes FS, Sennes LU, Carrau RL, Brito R, Ribas GC, Yasuda A, et al. Endoscopic anatomy of the pterygopalatine fossa and the transpterygoid approach: development of a surgical instruction model. Laryngoscope. 2008; 118:44–9.

39. Karci B, Midilli R, Erdogan U, Turhal G, Gode S. Endoscopic endonasal approach to the Vidian nerve and its relation to the surrounding structures: an anatomic cadaver study. Eur Arch Otorhinolaryngol. 2018; 275:2473–9.

40. DelGaudio JM. Endoscopic transnasal approach to the pterygopalatine fossa. Arch Otolaryngol Head Neck Surg. 2003; 129:441–6.

41. Hofstetter CP, Singh A, Anand VK, Kacker A, Schwartz TH. The endoscopic, endonasal, transmaxillary transpterygoid approach to the pterygopalatine fossa, infratemporal fossa, petrous apex, and the Meckel cave. J Neurosurg. 2010; 113:967–74.

42. Luginbuhl AJ, Campbell PG, Evans J, Rosen M. Endoscopic repair of high-flow cranial base defects using a bilayer button. Laryngoscope. 2010; 120:876–80.

43. Keshri A, Jain R, Manogaran RS, Behari S, Khatri D, Mathialagan A. Management of spontaneous CSF rhinorrhea: an institutional experience. J Neurol Surg B Skull Base. 2019; 80:493–9.

44. Chaskes MB, Fastenberg JH, Vimawala S, Nyquist GF, Rabinowitz MR, Chitguppi C, et al. A simple onlay sellar reconstruction does not increase the risk of postoperative cerebrospinal fluid leak in well-selected patients. J Neurol Surg B Skull Base. 2021; 82(Suppl 3):e231–5.

45. Wood J, Densky J, Boughter J, Sebelik M, Shires C. Anterior skull base reconstruction: does fat preparation matter? J Neurol Surg Rep. 2018; 79:e31–5.

46. Jeon C, Hong SD, Seol HJ, Lee JI, Nam DH, Hwang YJ, et al. Reconstructive outcome of intraoperative cerebrospinal fluid leak after endoscopic endonasal surgery for tumors involving skull base. J Clin Neurosci. 2017; 45:227–31.

47. Conger A, Zhao F, Wang X, Eisenberg A, Griffiths C, Esposito F, et al. Evolution of the graded repair of CSF leaks and skull base defects in endonasal endoscopic tumor surgery: trends in repair failure and meningitis rates in 509 patients. J Neurosurg. 2018; 130:861–75.

48. Soudry E, Turner JH, Nayak JV, Hwang PH. Endoscopic reconstruction of surgically created skull base defects: a systematic review. Otolaryngol Head Neck Surg. 2014; 150:730–8.

49. Karnezis TT, Baker AB, Soler ZM, Wise SK, Rereddy SK, Patel ZM, et al. Factors impacting cerebrospinal fluid leak rates in endoscopic sellar surgery. Int Forum Allergy Rhinol. 2016; 6:1117–25.

50. Harvey RJ, Parmar P, Sacks R, Zanation AM. Endoscopic skull base reconstruction of large dural defects: a systematic review of published evidence. Laryngoscope. 2012; 122:452–9.

51. Ahmed OH, Marcus S, Tauber JR, Wang B, Fang Y, Lebowitz RA. Efficacy of perioperative lumbar drainage following endonasal endoscopic cerebrospinal fluid leak repair. Otolaryngol Head Neck Surg. 2017; 156:52–60.

52. D’Anza B, Tien D, Stokken JK, Recinos PF, Woodard TR, Sindwani R. Role of lumbar drains in contemporary endonasal skull base surgery: meta-analysis and systematic review. Am J Rhinol Allergy. 2016; 30:430–5.

Fig. 1.
Extracapsular dissection. (A) After a wide visualization of the sella is achieved, the dura is sharply opened to expose the tumor inside. (B) A plane between the compressed “paper-thin” pituitary gland and the pseudocapsule is dissected in order to achieve extracapsular dissection.
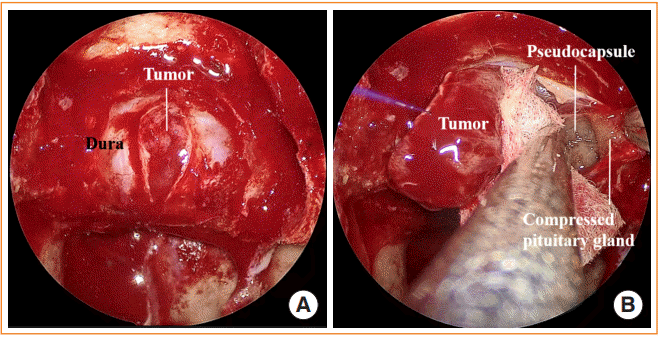
Fig. 2.
Cavernous sinus exploration. (A) Endoscopic view of the sella and the cavernous sinus (cadaver head) showing the pituitary gland, stalk, inferior parasellar ligament (IPL), and caroticoclinoid ligament (CCL). (B) Intraoperative photo after releasing the medial cavernous sinus wall exposing the cavernous internal carotid artery (ICA) inside. (C) Preoperative magnetic resonance image shows pituitary adenoma involving left cavernous sinus. (D) After opening the inferior intercavernous sinus, the medial cavernous sinus wall was dissected to gain access to the cavernous sinus in this patient. Paraclinoid ICA is identified inside the cavernous sinus.
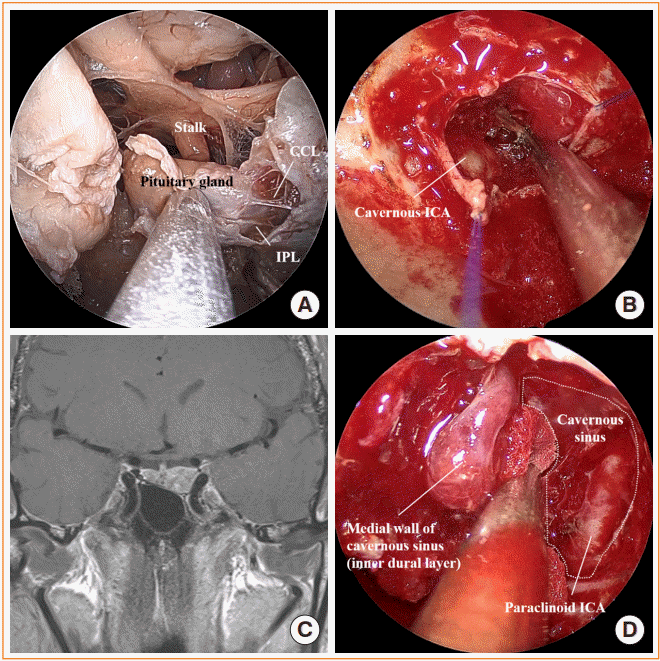
Fig. 3.
Removal of pituitary adenoma extending into the third ventricle. (A, B) Preoperative magnetic resonance image (MRI) shows giant pituitary adenoma extending superiorly into the third ventricle via tuber cinereum. (C, D) Postoperative MRI after removing the sellar and intraventricular tumor.
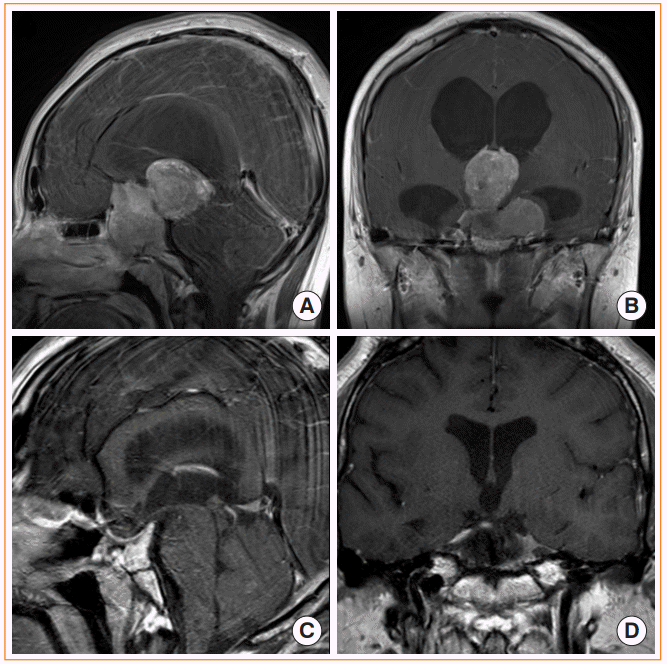
Fig. 4.
Endoscopic transtubercular/transplanar approach. (A) Preoperative magnetic resonance image (MRI) shows a pituitary adenoma extending anteriorly to cause bilateral frontal base and optic nerve compression. (B) Endoscopic view after removing the tumor shows the frontal lobe anterior cerebral artery complex covered by arachnoid membranes. (C) Postoperative computed tomography after gross total resection of the tumor. Hydroxyapatite cement was used to achieve rigid reconstruction (black asterisk). (D) Postoperative MRI after gross total resection of the tumor.
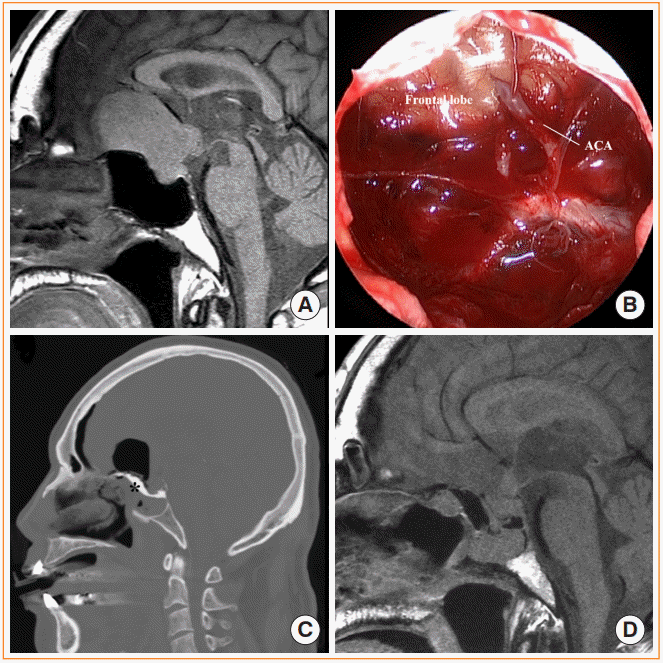
Fig. 5.
Craniotomy for pituitary adenoma with frontal lobe cuff. (A) Preoperative magnetic resonance image (MRI) shows pituitary adenoma with suprasellar extension and cuff of the frontal lobe (white asterisk). (B) Postoperative MRI shows the preserved pituitary stalk and gland. (C) Intraoperative photograph of the tumor via anterior interhemispheric approach. Lt, left; Rt, right.
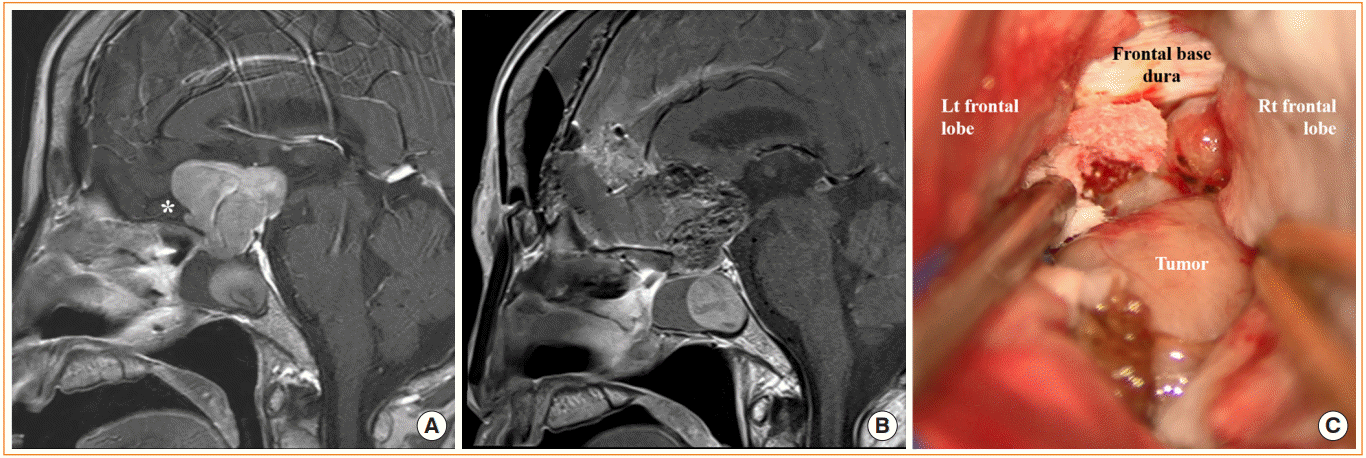
Fig. 6.
Endoscopic transclival approach. (A) Preoperative magnetic resonance image (MRI) shows a pituitary adenoma extending from the dorsum sellae to middle clivus. (B) Postoperative MRI after gross total resection of the tumor. Note that the sellar and clival portions of the skull base have been removed to access the tumor.
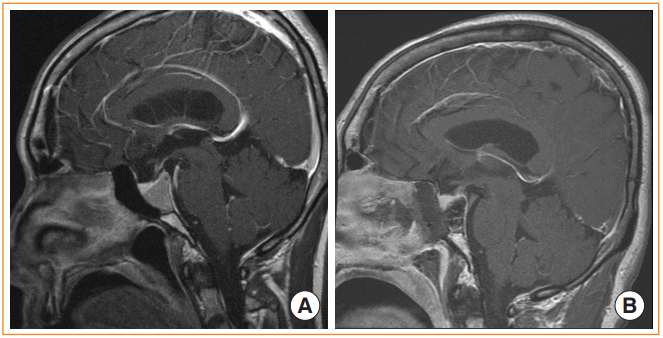
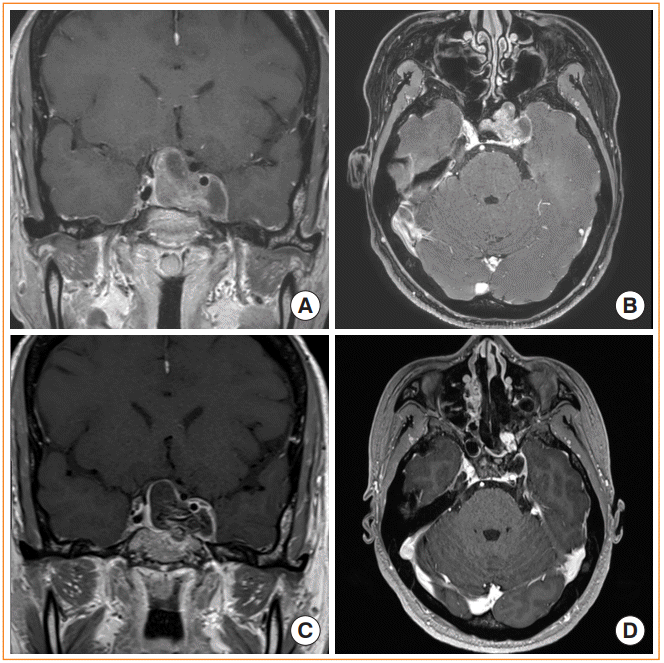
Fig. 7.
Endoscopic trans-pterygoid palatine fossa approach. (A, B) Preoperative magnetic resonance image (MRI) shows a pituitary adenoma invading into the lateral cavernous sinus. (C, D) Postoperative MRI after gross total resection of the tumor. The pterygopalatine fossa have been opened to access the lateral margin of the tumor. Nasoseptal flap was used for skull base reconstruction.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download