1. Lambert IH, Kristensen DM, Holm JB, Mortensen OH. Physiological role of taurine: from organism to organelle. Acta Physiol (Oxf). 2015; 213:191–212.
2. Ribeiro RA, Bonfleur ML, Batista TM, Borck PC, Carneiro EM. Regulation of glucose and lipid metabolism by the pancreatic and extra-pancreatic actions of taurine. Amino Acids. 2018; 50:1511–24.

3. Yu X, Hu L, Li S, Shen J, Wang D, Xu R, et al. Long non-coding RNA taurine upregulated gene 1 promotes osteosarcoma cell metastasis by mediating HIF-1α via miR-143-5p. Cell Death Dis. 2019; 10:280.

4. Tiedemann F, Gmelin L. Einige neue bestandtheile der galle des ochsen. Ann Phys. 1827; 85:326–37.

5. Demarcay H. Ueber die Natur der Galle. Ann Pharm. 1838; 27:270–91.

6. Jacobsen JG, Smith LH. Biochemistry and physiology of taurine and taurine derivatives. Physiol Rev. 1968; 48:424–511.

7. Kataoka H, Ohnishi N. Occurrence of taurine in plants. Agric Biol Chem. 2014; 50:1887–8.

8. Wishart DS, Feunang YD, Marcu A, Guo AC, Liang K, Vazquez-Fresno R, et al. HMDB 4.0: the human metabolome database for 2018. Nucleic Acids Res. 2018; 46(D1):D608–17.

9. Ma CC, Butler D, Milligan V, Hammann BA, Luo H, Brazdil JF, et al. Continuous process for the production of taurine from monoethanolamine. Ind Eng Chem Res. 2020; 59:13007–15.

10. Laidlaw SA, Grosvenor M, Kopple JD. The taurine content of common foodstuffs. JPEN J Parenter Enteral Nutr. 1990; 14:183–8.

11. Chawla D. Taurine and neonatal nutrition. Indian J Pediatr. 2018; 85:829.

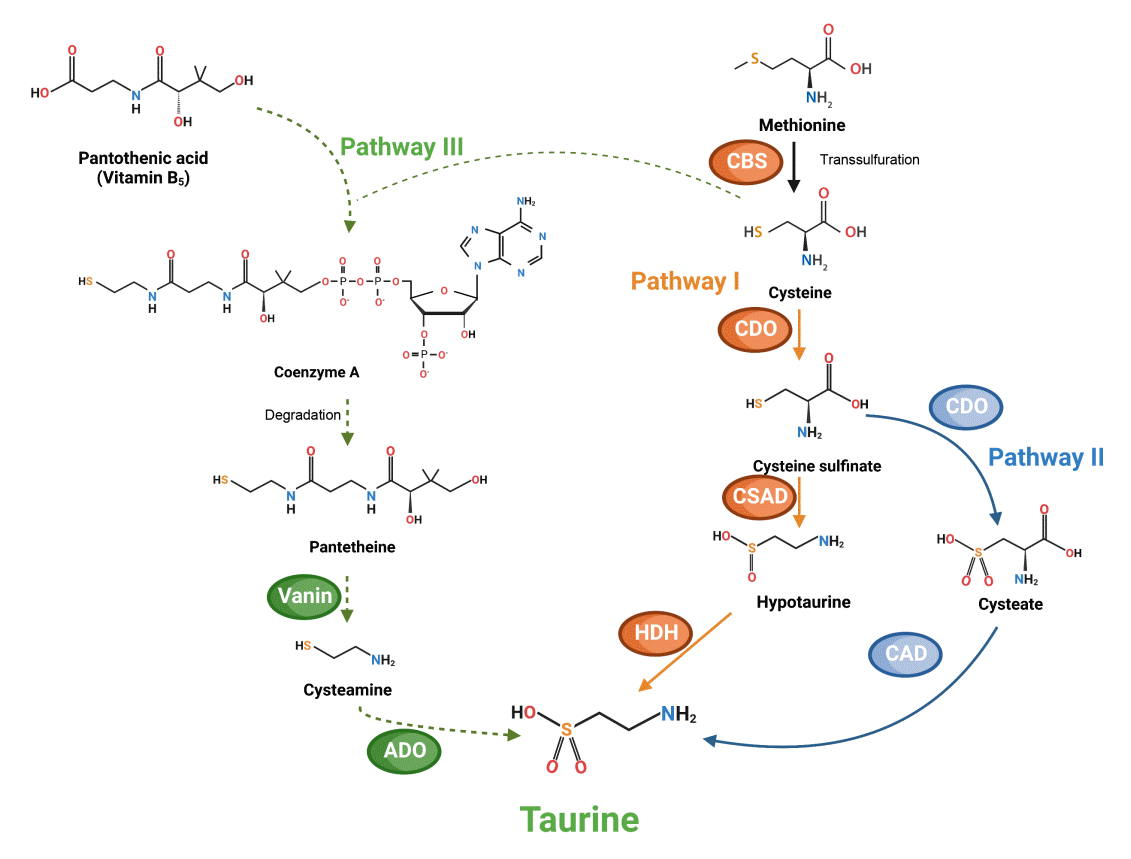
12. Tappaz ML. Taurine biosynthetic enzymes and taurine transporter: molecular identification and regulations. Neurochem Res. 2004; 29:83–96.

13. Sarenac TM, Mikov M. Bile acid synthesis: from nature to the chemical modification and synthesis and their applications as drugs and nutrients. Front Pharmacol. 2018; 9:939.
14. Huxtable RJ. Biochemistry of Sulfur. Boston: Springer;1986. Chapter 4, Taurine and the oxidative metabolism of cysteine. p. 121–97.
15. Anderson CM, Howard A, Walters JR, Ganapathy V, Thwaites DT. Taurine uptake across the human intestinal brush-border membrane is via two transporters: H+-coupled PAT1 (SLC36A1) and Na+- and Cl(-)-dependent TauT (SLC6A6). J Physiol. 2009; 587(Pt 4):731–44.
16. Lourenço R, Camilo ME. Taurine: a conditionally essential amino acid in humans?: an overview in health and disease. Nutr Hosp. 2002; 17:262–70.
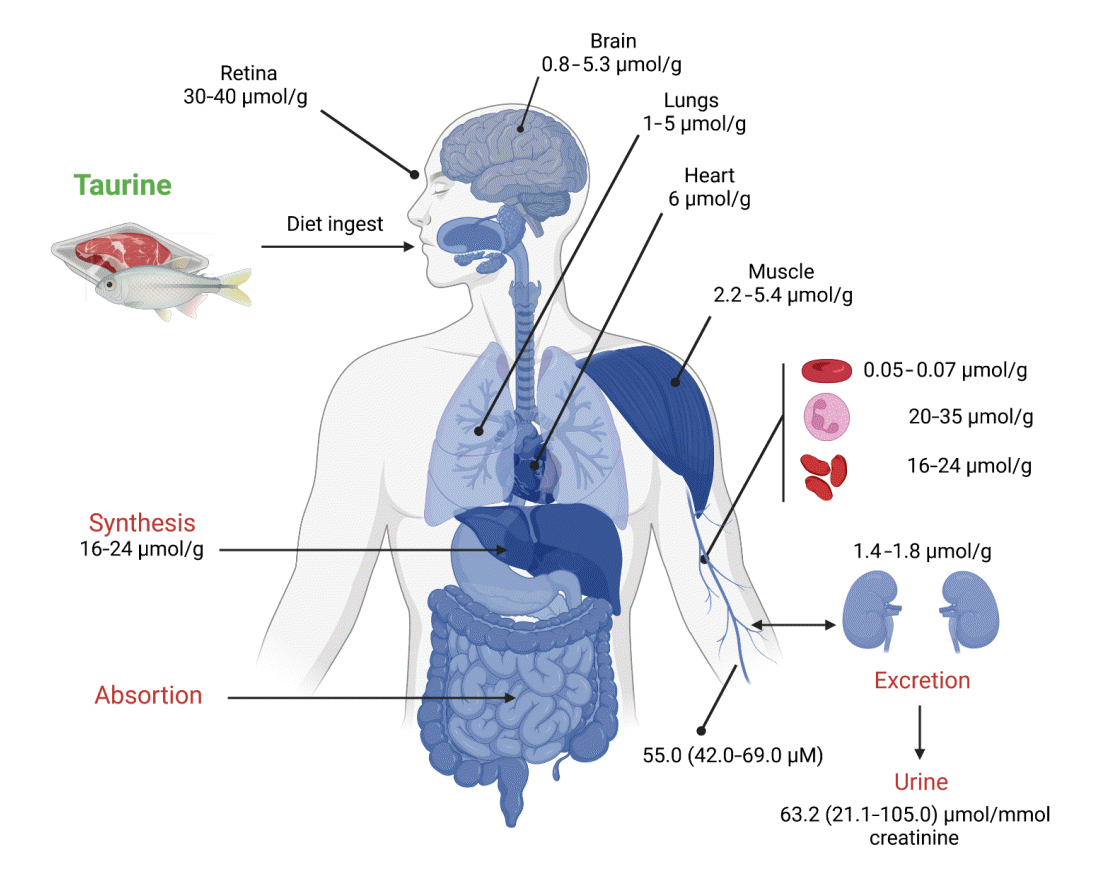
17. van Stijn MF, Vermeulen MA, Siroen MP, Wong LN, van den Tol MP, Ligthart-Melis GC, et al. Human taurine metabolism: fluxes and fractional extraction rates of the gut, liver, and kidneys. Metabolism. 2012; 61:1036–40.

18. Seidel U, Huebbe P, Rimbach G. Taurine: a regulator of cellular redox homeostasis and skeletal muscle function. Mol Nutr Food Res. 2019; 63:e1800569.

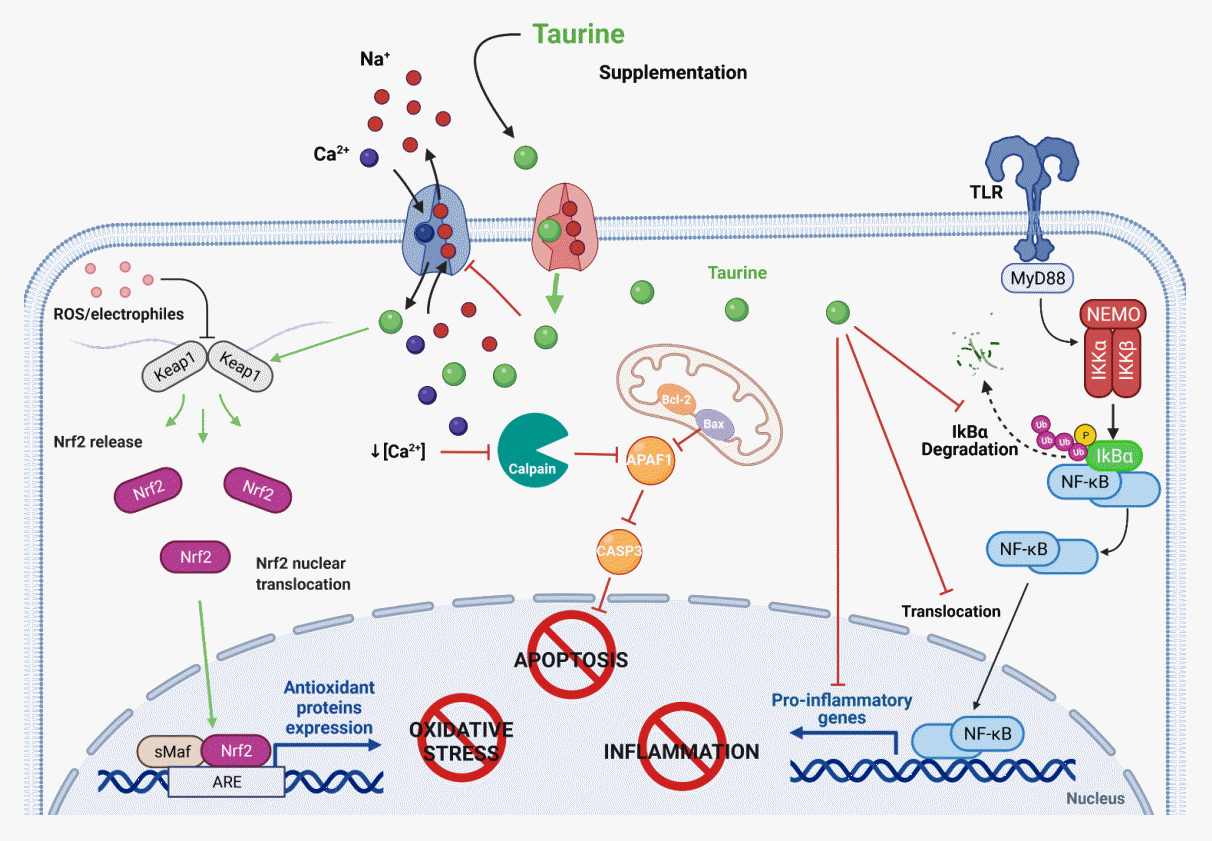
19. Jong CJ, Azuma J, Schaffer S. Mechanism underlying the antioxidant activity of taurine: prevention of mitochondrial oxidant production. Amino Acids. 2012; 42:2223–32.

20. Shimada K, Jong CJ, Takahashi K, Schaffer SW. Role of ROS production and turnover in the antioxidant activity of taurine. Adv Exp Med Biol. 2015; 803:581–96.

21. Maleki V, Mahdavi R, Hajizadeh-Sharafabad F, Alizadeh M. The effects of taurine supplementation on oxidative stress indices and inflammation biomarkers in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial. Diabetol Metab Syndr. 2020; 12:9.

22. Weiss SJ, Klein R, Slivka A, Wei M. Chlorination of taurine by human neutrophils: evidence for hypochlorous acid generation. J Clin Invest. 1982; 70:598–607.
23. Kang IS, Kim C. Taurine chloramine administered in vivo increases NRF2-regulated antioxidant enzyme expression in murine peritoneal macrophages. Adv Exp Med Biol. 2013; 775:259–67.

24. Schaffer SW, Shimada K, Jong CJ, Ito T, Azuma J, Takahashi K. Effect of taurine and potential interactions with caffeine on cardiovascular function. Amino Acids. 2014; 46:1147–57.

25. Bkaily G, Jazzar A, Normand A, Simon Y, Al-Khoury J, Jacques D. Taurine and cardiac disease: state of the art and perspectives. Can J Physiol Pharmacol. 2020; 98:67–73.

26. Ramila KC, Jong CJ, Pastukh V, Ito T, Azuma J, Schaffer SW. Role of protein phosphorylation in excitation-contraction coupling in taurine deficient hearts. Am J Physiol Heart Circ Physiol. 2015; 308:H232–9.

27. Dutka TL, Lamboley CR, Murphy RM, Lamb GD. Acute effects of taurine on sarcoplasmic reticulum Ca2+ accumulation and contractility in human type I and type II skeletal muscle fibers. J Appl Physiol (1985). 2014; 117:797–805.

28. Terrill JR, Pinniger GJ, Graves JA, Grounds MD, Arthur PG. Increasing taurine intake and taurine synthesis improves skeletal muscle function in the mdx mouse model for Duchenne muscular dystrophy. J Physiol. 2016; 594:3095–110.

29. Wu H, Jin Y, Wei J, Jin H, Sha D, Wu JY. Mode of action of taurine as a neuroprotector. Brain Res. 2005; 1038:123–31.

30. Jong CJ, Ito T, Prentice H, Wu JY, Schaffer SW. Role of mitochondria and endoplasmic reticulum in taurine-deficiency-mediated apoptosis. Nutrients. 2017; 9:795.

31. Roy A, Sil PC. Taurine protects murine hepatocytes against oxidative stress-induced apoptosis by tert-butyl hydroperoxide via PI3K/Akt and mitochondrial-dependent pathways. Food Chem. 2012; 131:1086–96.

32. Sun G, Wang X, Li T, Qu S, Sun J. Taurine attenuates acrylamide-induced apoptosis via a PI3K/AKT-dependent manner. Hum Exp Toxicol. 2018; 37:1249–57.

33. Li K, Wang D, Zhou X, Shao J, Li Y, Liu X, et al. Taurine protects against arsenic-induced apoptosis via PI3K/Akt pathway in primary cortical neurons. Adv Exp Med Biol. 2019; 1155:747–54.

34. Prideaux M, Kitase Y, Kimble M, O’Connell TM, Bonewald LF. Taurine, an osteocyte metabolite, protects against oxidative stress-induced cell death and decreases inhibitors of the Wnt/β-catenin signaling pathway. Bone. 2020; 137:115374.

35. Sun Q, Jia N, Yang J, Chen G. Nrf2 signaling pathway mediates the antioxidative effects of taurine against corticosterone-induced cell death in human SK-N-SH cells. Neurochem Res. 2018; 43:276–86.

36. Iezhitsa I, Agarwal R. New solutions for old challenges in glaucoma treatment: is taurine an option to consider? Neural Regen Res. 2021; 16:967–71.

37. Qiu T, Pei P, Yao X, Jiang L, Wei S, Wang Z, et al. Taurine attenuates arsenic-induced pyroptosis and nonalcoholic steatohepatitis by inhibiting the autophagic-inflammasomal pathway. Cell Death Dis. 2018; 9:946.

38. Gentile CL, Nivala AM, Gonzales JC, Pfaffenbach KT, Wang D, Wei Y, et al. Experimental evidence for therapeutic potential of taurine in the treatment of nonalcoholic fatty liver disease. Am J Physiol Regul Integr Comp Physiol. 2011; 301:R1710–22.

39. Wu G, Yang J, Lv H, Jing W, Zhou J, Feng Y, et al. Taurine prevents ethanol-induced apoptosis mediated by mitochondrial or death receptor pathways in liver cells. Amino Acids. 2018; 50:863–75.

40. Wen C, Li F, Zhang L, Duan Y, Guo Q, Wang W, et al. Taurine is involved in energy metabolism in muscles, adipose tissue, and the liver. Mol Nutr Food Res. 2019; 63:e1800536.

41. Liu Y, Li F, Zhang L, Wu J, Wang Y, Yu H. Taurine alleviates lipopolysaccharide induced liver injury by anti inflammation and antioxidants in rats. Mol Med Rep. 2017; 16:6512–7.
42. De Luca A, Pierno S, Camerino DC. Taurine: the appeal of a safe amino acid for skeletal muscle disorders. J Transl Med. 2015; 13:243.

43. Kp AD, Martin A. Recent insights into the molecular regulators and mechanisms of taurine to modulate lipid metabolism: a review. Crit Rev Food Sci Nutr. 2022; Jan. 18. [Epub].
https://doi.org/10.1080/10408398.2022.2026873.

44. Midwinter RG, Cheah FC, Moskovitz J, Vissers MC, Winterbourn CC. IkappaB is a sensitive target for oxidation by cell-permeable chloramines: inhibition of NF-kappaB activity by glycine chloramine through methionine oxidation. Biochem J. 2006; 396:71–8.
45. Gomez-Chavez F, Correa D, Navarrete-Meneses P, Cancino-Diaz JC, Cancino-Diaz ME, Rodriguez-Martinez S. NF- κB and its regulators during pregnancy. Front Immunol. 2021; 12:679106.
46. Niu X, Zheng S, Liu H, Li S. Protective effects of taurine against inflammation, apoptosis, and oxidative stress in brain injury. Mol Med Rep. 2018; 18:4516–22.

47. Ahmadi S, Wang S, Nagpal R, Wang B, Jain S, Razazan A, et al. A human-origin probiotic cocktail ameliorates aging-related leaky gut and inflammation via modulating the microbiota/taurine/tight junction axis. JCI Insight. 2020; 5:e132055.

48. Kim SH, Yum HW, Kim SH, Kim SJ, Kim K, Kim C, et al. Topically applied taurine chloramine protects against UVB-induced oxidative stress and inflammation in mouse skin. Antioxidants (Basel). 2021; 10:867.

49. Ramos CO, Campos KK, Costa GP, Cangussu SD, Talvani A, Bezerra FS. Taurine treatment decreases inflammation and oxidative stress in lungs of adult mice exposed to cigarette smoke. Regul Toxicol Pharmacol. 2018; 98:50–7.

50. Abd-Elhakim YM, Ghoneim MH, Ebraheim LL, Imam TS. Taurine and hesperidin rescues carbon tetrachloride-triggered testicular and kidney damage in rats via modulating oxidative stress and inflammation. Life Sci. 2020; 254:117782.

51. Wang G, Ma N, He F, Kawanishi S, Kobayashi H, Oikawa S, et al. Taurine attenuates carcinogenicity in ulcerative colitis-colorectal cancer mouse model. Oxid Med Cell Longev. 2020; 2020:7935917.

52. Wu L, Zhang Q, Li Y, Song W, Chen A, Liu J, et al. Collagen sponge prolongs taurine release for improved wound healing through inflammation inhibition and proliferation stimulation. Ann Transl Med. 2021; 9:1010.

53. Faghfouri AH, Seyyed Shoura SM, Fathollahi P, Shadbad MA, Papi S, Ostadrahimi A, et al. Profiling inflammatory and oxidative stress biomarkers following taurine supplementation: a systematic review and dose-response meta-analysis of controlled trials. Eur J Clin Nutr. 2022; 76:647–58.

54. Pasantes-Morales H. Taurine homeostasis and volume control. Adv Neurobiol. 2017; 16:33–53.

55. Shennan DB. Swelling-induced taurine transport: relationship with chloride channels, anion-exchangers and other swelling-activated transport pathways. Cell Physiol Biochem. 2008; 21:15–28.

56. Netti V, Pizzoni A, Perez-Dominguez M, Ford P, Pasantes-Morales H, Ramos-Mandujano G, et al. Release of taurine and glutamate contributes to cell volume regulation in human retinal Muller cells: differences in modulation by calcium. J Neurophysiol. 2018; 120:973–84.
57. Pasantes-Morales H, Quesada O, Moran J. Taurine: an osmolyte in mammalian tissues. Adv Exp Med Biol. 1998; 442:209–17.

58. Morgan EF, Unnikrisnan GU, Hussein AI. Bone mechanical properties in healthy and diseased states. Annu Rev Biomed Eng. 2018; 20:119–43.

59. Allen MR, Burr DB. Basic and applied bone biology. San Diego: Elsevier;2019. Chapter 1, Bone morphology and organization. p. 3–26.
60. Allen MR, Burr DB. Basic and applied bone biology. San Diego: Elsevier;2019. Chapter 5, Bone growth, modeling, and remodeling. p. 85–100.
61. Aghajanian P, Mohan S. The art of building bone: emerging role of chondrocyte-to-osteoblast transdifferentiation in endochondral ossification. Bone Res. 2018; 6:19.

62. Suzuki A, Iwata J. Amino acid metabolism and autophagy in skeletal development and homeostasis. Bone. 2021; 146:115881.

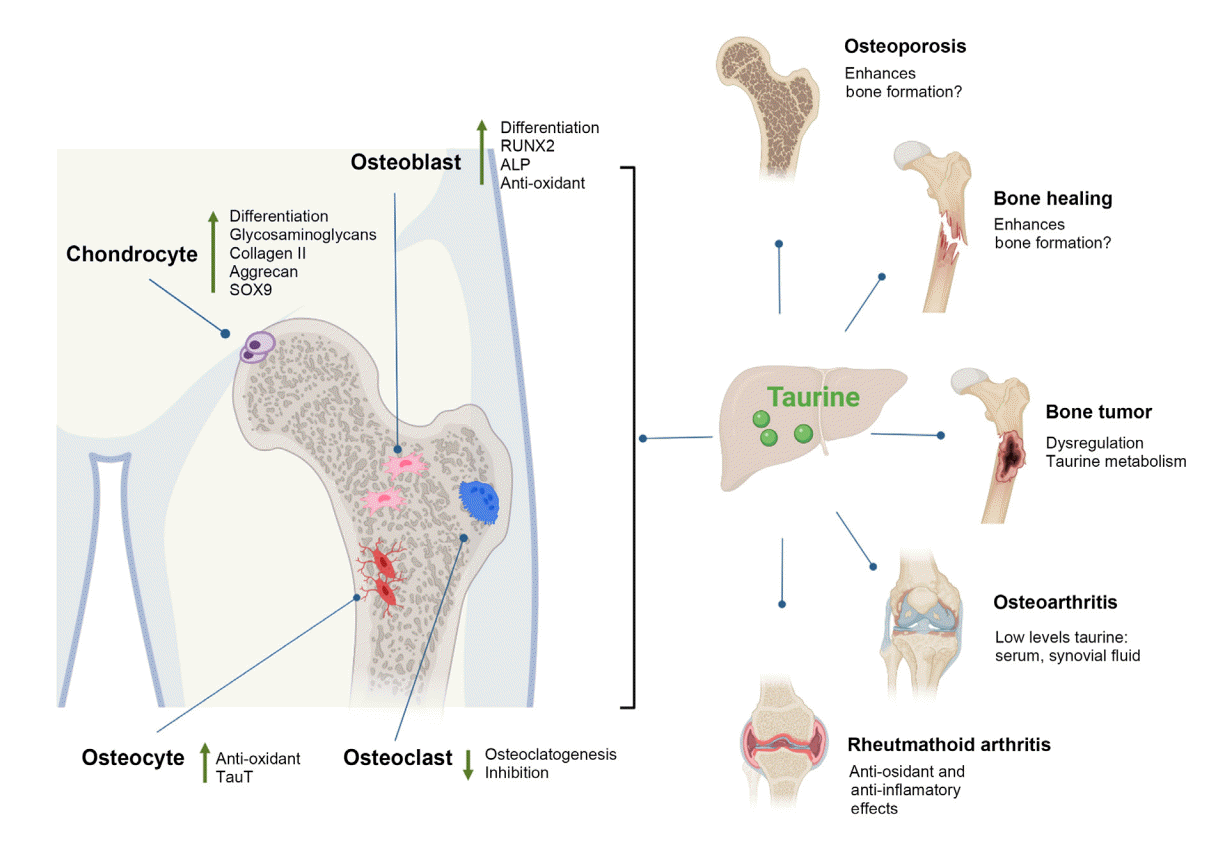
63. Kim SJ, Lee HW, Gupta RC. Taurine, bone growth and bone development. Curr Nutr Food Sci. 2008; 4:135–44.

64. Roman-Garcia P, Quiros-Gonzalez I, Mottram L, Lieben L, Sharan K, Wangwiwatsin A, et al. Vitamin B12-dependent taurine synthesis regulates growth and bone mass. J Clin Invest. 2014; 124:2988–3002.

65. Shari FH, Dawood H, Hassan JK, Aljazeari QA, Najm MA, Salahuddin A, et al. To study the effect of taurine on the effects of vital bones and regulate the level of glucose in type II diabetes. Int J Res Pharm Sci. 2019; 10:2545–51.

66. Moon PD, Kim MH, Lim HS, Oh HA, Nam SY, Han NR, et al. Taurine, a major amino acid of oyster, enhances linear bone growth in a mouse model of protein malnutrition. Biofactors. 2015; 41:190–7.

67. Choi MJ, Seo JN. Effect of taurine feeding on bone mineral density and bone markers in rats. Adv Exp Med Biol. 2013; 776:51–8.

68. Modupe OS, Adijat BR, Salau AK. Immune response and bone marker enzyme activities of broiler birds fed graded level taurine-supplemented-diets. Ann Sci Technol. 2021; 6:16–25.

69. Martiniakova M, Sarocka A, Babosova R, Galbavy D, Kapusta E, Goc Z, et al. Bone microstructure of mice after prolonged taurine treatment. Physiol Res. 2019; 68:519–23.

70. Rutkovskiy A, Stenslokken KO, Vaage IJ. Osteoblast differentiation at a glance. Med Sci Monit Basic Res. 2016; 22:95–106.

71. Long F. Building strong bones: molecular regulation of the osteoblast lineage. Nat Rev Mol Cell Biol. 2011; 13:27–38.

72. Park M, Choi HK, An JH. Taurine activates BMP-2/Wnt3a-mediated osteoblast differentiation and mineralization via Akt and MAPK signaling. Iran J Public Health. 2019; 48:1960–70.

73. Zhou C, Zhang X, Xu L, Wu T, Cui L, Xu D. Taurine promotes human mesenchymal stem cells to differentiate into osteoblast through the ERK pathway. Amino Acids. 2014; 46:1673–80.

74. Lou J, Han D, Yu H, Yu G, Jin M, Kim SJ. Cytoprotective effect of taurine against hydrogen peroxide-induced oxidative stress in UMR-106 cells through the Wnt/β-catenin signaling pathway. Biomol Ther (Seoul). 2018; 26:584–90.
75. Zhang LY, Zhou YY, Chen F, Wang B, Li J, Deng YW, et al. Taurine inhibits serum deprivation-induced osteoblast apoptosis via the taurine transporter/ERK signaling pathway. Braz J Med Biol Res. 2011; 44:618–23.

76. Yuan LQ, Liu W, Cui RR, Wang D, Meng JC, Xie H, et al. Taurine inhibits osteoclastogenesis through the taurine transporter. Amino Acids. 2010; 39:89–99.

77. Kang YS, Kim SJ. The change of taurine transport in osteocytes by oxidative stress, hypertonicity and calcium channel blockers. Biomol Ther. 2008; 16:219–25.

78. Ono T, Nakashima T. Recent advances in osteoclast biology. Histochem Cell Biol. 2018; 149:325–41.

79. Park-Min KH. Mechanisms involved in normal and pathological osteoclastogenesis. Cell Mol Life Sci. 2018; 75:2519–28.

80. Lee NK, Choi YG, Baik JY, Han SY, Jeong DW, Bae YS, et al. A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood. 2005; 106:852–9.

81. Jang HJ, Kim SJ. Taurine exerts anti-osteoclastogenesis activity via inhibiting ROS generation, JNK phosphorylation and COX-2 expression in RAW264.7 cells. J Recept Signal Transduct Res. 2013; 33:387–91.

82. Kang IS, Kim C. Taurine chloramine inhibits osteoclastic differentiation and osteoclast marker expression in RAW 264.7 cells. Adv Exp Med Biol. 2019; 1155:61–70.

83. Kim C, Kang IS. Taurine chloramine, a taurine metabolite from activated neutrophils, inhibits osteoclastogenesis by suppressing NFATc1 expression. Adv Exp Med Biol. 2015; 803:99–107.

84. Goldring SR. The osteocyte: key player in regulating bone turnover. RMD Open. 2015; 1(Suppl 1):e000049.

85. Robling AG, Bonewald LF. The osteocyte: new insights. Annu Rev Physiol. 2020; 82:485–506.

86. Einhorn TA, Gerstenfeld LC. Fracture healing: mechanisms and interventions. Nat Rev Rheumatol. 2015; 11:45–54.

87. Kaya Bicer E, Yener N, Doganavsargil B, Argin M, Kapubagli A. Does taurıne improve fracture healing? An experimental study. J Ist Faculty Med. 2018; 81:91–8.
88. Hirata H, Ueda S, Ichiseki T, Shimasaki M, Ueda Y, Kaneuji A, et al. Taurine inhibits glucocorticoid-induced bone mitochondrial injury, preventing osteonecrosis in rabbits and cultured osteocytes. Int J Mol Sci. 2020; 21:6892.

89. Yang SS, Oh JM, Chun S, Kim BS, Kim CS, Lee J. Tauroursodeoxycholic acid induces angiogenic activity in endothelial cells and accelerates bone regeneration. Bone. 2020; 130:115073.

90. Samadian H, Farzamfar S, Vaez A, Ehterami A, Bit A, Alam M, et al. A tailored polylactic acid/polycaprolactone biodegradable and bioactive 3D porous scaffold containing gelatin nanofibers and taurine for bone regeneration. Sci Rep. 2020; 10:13366.

91. Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Int. 1994; 4:368–81.
92. Hershman SH, Razi AE. Vertebral compression fractures in osteoporotic and pathologic bone. Cham: Springer;2020. Chapter 3, The economic burden of osteoporosis. p. 21–9.

93. Pontes TA, Barbosa AD, Silva RD, Melo-Junior MR, Silva RO. Osteopenia-osteoporosis discrimination in postmenopausal women by 1H NMR-based metabonomics. PLoS One. 2019; 14:e0217348.

94. Zhao Q, Shen H, Su KJ, Zhang JG, Tian Q, Zhao LJ, et al. Metabolomic profiles associated with bone mineral density in US Caucasian women. Nutr Metab (Lond). 2018; 15:57.

95. Miyamoto T, Hirayama A, Sato Y, Koboyashi T, Katsuyama E, Kanagawa H, et al. Metabolomics-based profiles predictive of low bone mass in menopausal women. Bone Rep. 2018; 9:11–8.

96. Cheong SH, Chang KJ. The preventive effect of fermented milk supplement containing tomato (lycopersion esculentum) and taurine on bone loss in ovariectomized rats. Adv Exp Med Biol. 2009; 643:333–40.

97. Yu L, Qi H, An G, Bao J, Ma B, Zhu J, et al. Association between metabolic profiles in urine and bone mineral density of pre- and postmenopausal Chinese women. Menopause. 2019; 26:94–102.

98. Berry TM, Moustafa AA. Osteoporosis and the effect of dysregulation of the transsulfuration pathway via taurine on intracellular calcium homeostasis, vitamin D absorption and vitamin K absorption. Clin Nutr ESPEN. 2021; 43:191–6.

99. Ye M, Zhang C, Jia W, Shen Q, Qin X, Zhang H, et al. Metabolomics strategy reveals the osteogenic mechanism of yak (Bos grunniens) bone collagen peptides on ovariectomy-induced osteoporosis in rats. Food Funct. 2020; 11:1498–512.
100. Chen SY, Yu HT, Kao JP, Yang CC, Chiang SS, Mishchuk DO, et al. An NMR metabolomic study on the effect of alendronate in ovariectomized mice. PLoS One. 2014; 9:e106559.

101. Liu S, Yuan X, Ma C, Zhao J, Xiong Z. 1H-NMR-based urinary metabolomic analysis for the preventive effects of gushudan on glucocorticoid-induced osteoporosis rats. Anal Biochem. 2020; 610:113992.

102. Ahmed HH, Hamza AH. Potential role of arginine, glutamine and taurine in ameliorating osteoporotic biomarkers in ovariectomized rats. Rep Opin. 2009; 1:24–35.
103. Paulos J, Poitout DG. Bone tumors. London: Springer;2021. Chapter 2, Osteomas. p. 19–20.

104. Lv D, Zou Y, Zeng Z, Yao H, Ding S, Bian Y, et al. Comprehensive metabolomic profiling of osteosarcoma based on UHPLC-HRMS. Metabolomics. 2020; 16:120.

105. Lopez-Garrido L, Banuelos-Hernandez AE, Perez-Hernandez E, Tecualt-Gomez R, Quiroz-Williams J, Ariza-Castolo A, et al. Metabolic profiling of serum in patients with cartilage tumours using 1 H-NMR spectroscopy: a pilot study. Magn Reson Chem. 2020; 58:65–76.

106. Duarte IF, Lamego I, Marques J, Marques MP, Blaise BJ, Gil AM. Nuclear magnetic resonance (NMR) study of the effect of cisplatin on the metabolic profile of MG-63 osteosarcoma cells. J Proteome Res. 2010; 9:5877–86.

107. Marley K, Helfand SC, Edris WA, Mata JE, Gitelman AI, Medlock J, et al. The effects of taurolidine alone and in combination with doxorubicin or carboplatin in canine osteosarcoma in vitro. BMC Vet Res. 2013; 9:15.

108. Pilz M, Holinka J, Vavken P, Marian B, Krepler P. Taurine chloramine induces apoptosis in human osteosarcoma cell lines. J Orthop Res. 2012; 30:2046–51.

109. Green JD, Tollemar V, Dougherty M, Yan Z, Yin L, Ye J, et al. Multifaceted signaling regulators of chondrogenesis: implications in cartilage regeneration and tissue engineering. Genes Dis. 2015; 2:307–27.

110. Yao X, Huang H, Li Z, Liu X, Fan W, Wang X, et al. Taurine promotes the cartilaginous differentiation of human umbilical cord-derived mesenchymal stem cells in vitro. Neurochem Res. 2017; 42:2344–53.

111. Liu Q, Lu Z, Wu H, Zheng L. Chondroprotective effects of taurine in primary cultures of human articular chondrocytes. Tohoku J Exp Med. 2015; 235:201–13.

112. Karjalainen HM, Qu C, Leskela SS, Rilla K, Lammi MJ. Chondrocytic cells express the taurine transporter on their plasma membrane and regulate its expression under anisotonic conditions. Amino Acids. 2015; 47:561–70.

113. Peat G, Thomas MJ. Osteoarthritis year in review 2020: epidemiology & therapy. Osteoarthritis Cartilage. 2021; 29:180–9.

114. Mobasheri A, Rayman MP, Gualillo O, Sellam J, van der Kraan P, Fearon U. The role of metabolism in the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2017; 13:302–11.

115. Chen R, Han S, Liu X, Wang K, Zhou Y, Yang C, et al. Perturbations in amino acids and metabolic pathways in osteoarthritis patients determined by targeted metabolomics analysis. J Chromatogr B Analyt Technol Biomed Life Sci. 2018; 1085:54–62.

116. Yang G, Zhang H, Chen T, Zhu W, Ding S, Xu K, et al. Metabolic analysis of osteoarthritis subchondral bone based on UPLC/Q-TOF-MS. Anal Bioanal Chem. 2016; 408:4275–86.

117. Anderson JR, Chokesuwattanaskul S, Phelan MM, Welting TJM, Lian LY, Peffers MJ, et al. 1H NMR metabolomics identifies underlying inflammatory pathology in osteoarthritis and rheumatoid arthritis synovial joints. J Proteome Res. 2018; 17:3780–90.
118. Xu Z, Chen T, Luo J, Ding S, Gao S, Zhang J. Cartilaginous metabolomic study reveals potential mechanisms of osteophyte formation in osteoarthritis. J Proteome Res. 2017; 16:1425–35.

119. Saraswati AAEW, Sunardi D, Lubis AMT, Heru F, Mudjihartini N. Taurine intakes increase superoxide dismutase activity in knee osteoarthritis. IOP Conf Ser Earth Environ Sci. 2019; 217:012054.

120. Bian Y, Zhang M, Wang K. Taurine protects against knee osteoarthritis development in experimental rat models. Knee. 2018; 25:374–80.

121. Bian Y, Wang H, Sun S. Taurine alleviates endoplasmic reticulum stress in the chondrocytes from patients with osteoarthritis. Redox Rep. 2018; 23:118–24.

122. Almutairi K, Nossent J, Preen D, Keen H, Inderjeeth C. The global prevalence of rheumatoid arthritis: a meta-analysis based on a systematic review. Rheumatol Int. 2021; 41:863–77.

123. Liu Y, Wei M, Yue K, Wang R, Ma Y, Men L, et al. Non-target metabonomic method provided new insights on the therapeutical mechanism of Gancao Fuzi decoction on rheumatoid arthritis rats. J Chromatogr B Analyt Technol Biomed Life Sci. 2019; 1105:93–103.

124. Cheng B, Zheng H, Wu F, Wu J, Liu X, Tang C, et al. Metabolomics analysis of Danggui Sini decoction on treatment of collagen-induced arthritis in rats. J Chromatogr B Analyt Technol Biomed Life Sci. 2017; 1061-1062:282–91.

125. Yun X, Dong S, Hu Q, Dai Y, Xia Y. 1H NMR-based metabolomics approach to investigate the urine samples of collagen-induced arthritis rats and the intervention of tetrandrine. J Pharm Biomed Anal. 2018; 154:302–11.

126. Sweeney SR, Kavanaugh A, Lodi A, Wang B, Boyle D, Tiziani S, et al. Metabolomic profiling predicts outcome of rituximab therapy in rheumatoid arthritis. RMD Open. 2016; 2:e000289.

127. Wang Z, Chen Z, Yang S, Wang Y, Yu L, Zhang B, et al. (1) H NMR-based metabolomic analysis for identifying serum biomarkers to evaluate methotrexate treatment in patients with early rheumatoid arthritis. Exp Ther Med. 2012; 4:165–71.
128. Malek Mahdavi A, Javadivala Z. A systematic review of preclinical studies on the efficacy of taurine for the treatment of rheumatoid arthritis. Amino Acids. 2021; 53:783–800.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download