1. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020; 323(20):2052–2059. PMID:
32320003.

2. Bourgonje AR, Abdulle AE, Timens W, Hillebrands JL, Navis GJ, Gordijn SJ, et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J Pathol. 2020; 251(3):228–248. PMID:
32418199.

3. Bwire GM. Coronavirus: why men are more vulnerable to Covid-19 than women? SN Compr Clin Med. 2020; 2(7):874–876. PMID:
32838138.

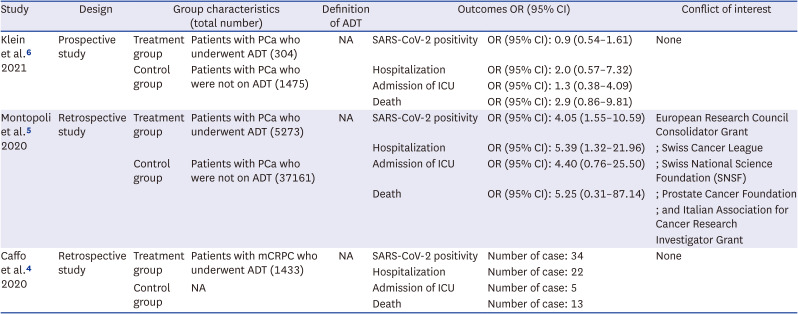
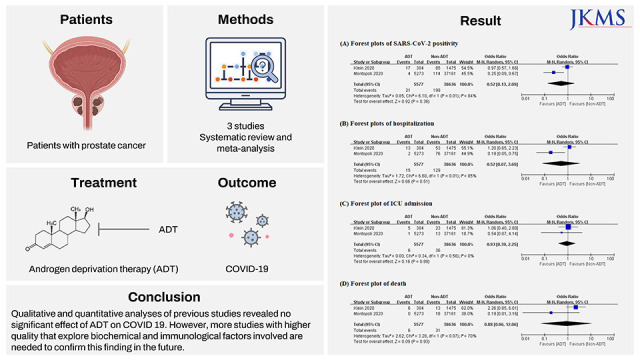
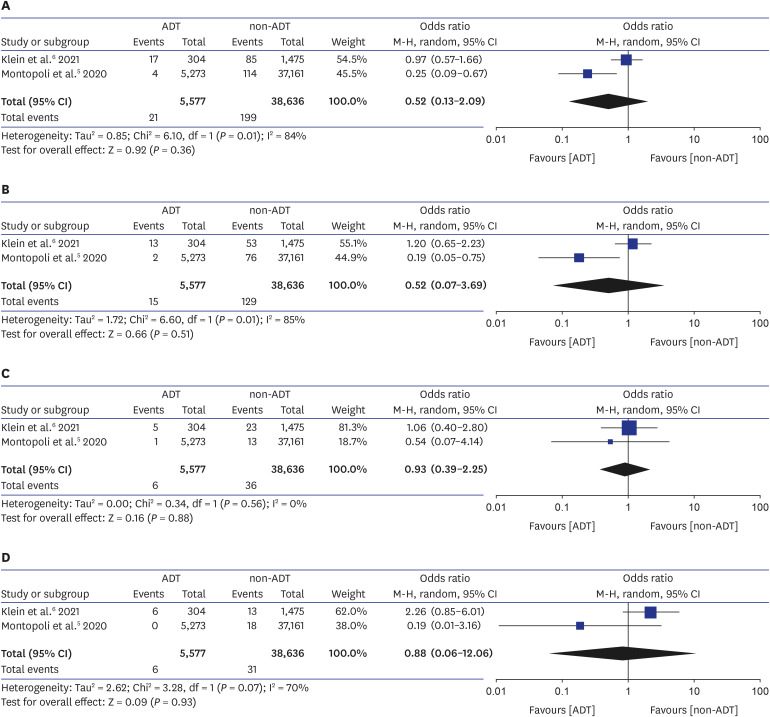
4. Caffo O, Zagonel V, Baldessari C, Berruti A, Bortolus R, Buti S, et al. On the relationship between androgen-deprivation therapy for prostate cancer and risk of infection by SARS-CoV-2. Ann Oncol. 2020; 31(10):1415–1416. PMID:
32562741.

5. Montopoli M, Zumerle S, Vettor R, Rugge M, Zorzi M, Catapano CV, et al. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532). Ann Oncol. 2020; 31(8):1040–1045. PMID:
32387456.

6. Klein EA, Li J, Milinovich A, Schold JD, Sharifi N, Kattan MW, et al. Androgen deprivation therapy in men with prostate cancer does not affect risk of infection with SARS-CoV-2. J Urol. 2021; 205(2):441–443. PMID:
32897764.

7. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015; 4(1):1. PMID:
25554246.

8. Sterne JA, Hernán MA, McAleenan A, Reeves BC, Higgins JPT. Assessing risk of bias in a non-randomized study. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions. Chichester, England: John Wiley & Sons Ltd;2019. p. 621–641.
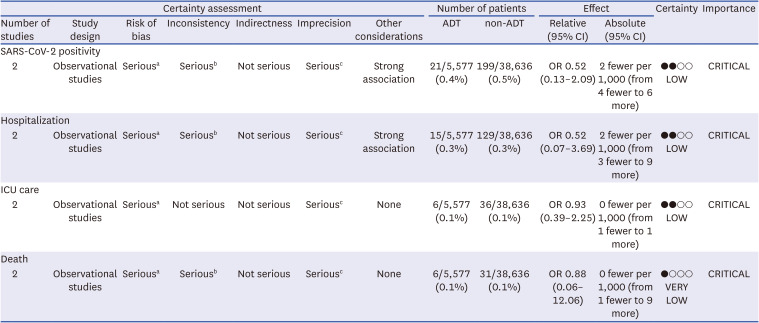
9. Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011; 64(4):383–394. PMID:
21195583.

10. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011; 343:d5928. PMID:
22008217.

11. Zhou F, Gao S, Han D, Han W, Chen S, Patalano S, et al. TMPRSS2-ERG activates NO-cGMP signaling in prostate cancer cells. Oncogene. 2019; 38(22):4397–4411. PMID:
30718921.

12. Zoni E, Karkampouna S, Thalmann GN, Kruithof-de Julio M, Spahn M. Emerging aspects of microRNA interaction with TMPRSS2-ERG and endocrine therapy. Mol Cell Endocrinol. 2018; 462(Pt A):9–16. PMID:
28189568.

13. Bennani NN, Bennani-Baiti IM. Androgen deprivation therapy may constitute a more effective COVID-19 prophylactic than therapeutic strategy. Ann Oncol. 2020; 31(11):1585–1586. PMID:
32822832.

14. Cohn BA, Cirillo PM, Hopper BR, Siiteri PK. Third trimester estrogens and maternal breast cancer: prospective evidence. J Clin Endocrinol Metab. 2017; 102(10):3739–3748. PMID:
28973345.

15. Channappanavar R, Fett C, Mack M, Ten Eyck PP, Meyerholz DK, Perlman S. Sex-based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. J Immunol. 2017; 198(10):4046–4053. PMID:
28373583.

16. Al-Lami RA, Urban RJ, Volpi E, Algburi AM, Baillargeon J. Sex hormones and novel corona virus infectious disease (COVID-19). Mayo Clin Proc. 2020; 95(8):1710–1714. PMID:
32753145.

17. Cutolo M, Smith V, Paolino S. Understanding immune effects of oestrogens to explain the reduced morbidity and mortality in female versus male COVID-19 patients. Comparisons with autoimmunity and vaccination. Clin Exp Rheumatol. 2020; 38(3):383–386. PMID:
32452350.
18. Moreno-Eutimio MA, López-Macías C, Pastelin-Palacios R. Bioinformatic analysis and identification of single-stranded RNA sequences recognized by TLR7/8 in the SARS-CoV-2, SARS-CoV, and MERS-CoV genomes. Microbes Infect. 2020; 22(4-5):226–229. PMID:
32361001.

19. Silva-Cardoso SC, Affandi AJ, Spel L, Cossu M, van Roon JA, Boes M, et al. CXCL4 exposure potentiates TLR-driven polarization of human monocyte-derived dendritic cells and increases stimulation of T cells. J Immunol. 2017; 199(1):253–262. PMID:
28515281.

20. Raisi-Estabragh Z, McCracken C, Ardissino M, et al. Non-white ethnicity, male sex, and higher body mass index, but not medications acting on the renin-angiotensin system are associated with coronavirus disease 2019 (COVID-19) hospitalisation: review of the first 669 cases from the UK Biobank. MedRxiv. 2020; 05. 15. DOI:
10.1101/2020.05.10.20096925.

21. Montaño LM, Espinoza J, Flores-Soto E, Chávez J, Perusquía M. Androgens are bronchoactive drugs that act by relaxing airway smooth muscle and preventing bronchospasm. J Endocrinol. 2014; 222(1):1–13. PMID:
24781253.

22. Mohan SS, Knuiman MW, Divitini ML, James AL, Musk AW, Handelsman DJ, et al. Higher serum testosterone and dihydrotestosterone, but not oestradiol, are independently associated with favourable indices of lung function in community-dwelling men. Clin Endocrinol (Oxf). 2015; 83(2):268–276. PMID:
25660119.

23. Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Baltimore Longitudinal Study of Aging. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. J Clin Endocrinol Metab. 2001; 86(2):724–731. PMID:
11158037.

24. Rastrelli G, Di Stasi V, Inglese F, Beccaria M, Garuti M, Di Costanzo D, et al. Low testosterone levels predict clinical adverse outcomes in SARS-CoV-2 pneumonia patients. Andrology. 2021; 9(1):88–98. PMID:
32436355.






 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download