INTRODUCTION
METHODS
Animals
Experimental design
Tissue homogenization
Immunoassay of DNA adducts
Histological examination
Interleukin (IL)-6 and tumor necrosis factor (TNF)-α in mouse serum
Western blot and immunohistochemical staining
Data analysis
Ethics statement
RESULTS
Immunoassay of DNA adducts
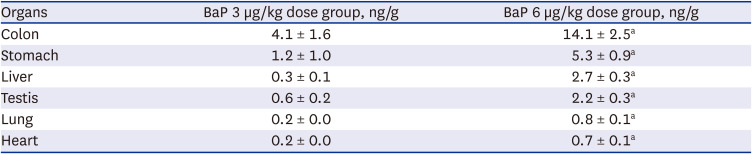
Table 1
Comparison of BaP−DNA adduct formation on main types of tissues following 3 and 6 µg/kg BaP of oral administration for 60 days
Histological examination
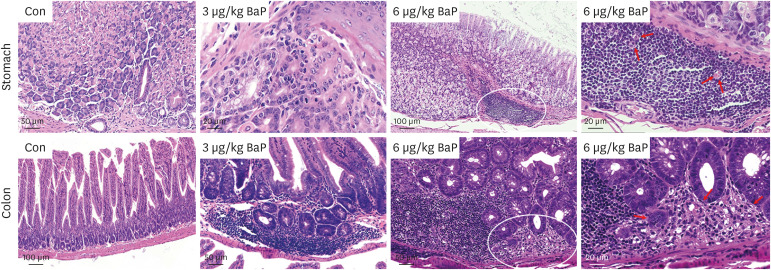 | Fig. 1Histopathological changes in the stomach and colon of mice after daily oral intake of 3 or 6 µg/kg BaP for 60 days, respectively. No histologic damage in the stomach and colon is observed in mice with vehicle controls. A large number of cell infiltration and inflammatory cells of the stomach, parts of the crypts cells destroyed, and the surface epithelium damaged in the colon are observed following oral intake of 3 µg/kg BaP, respectively. The inflammatory cells are induced by oral intake of 6 µg/kg BaP. The mononuclear cells, and multifocal cells in the stomach and the colon are induced following oral intake of 6 µg/kg BaP, in which the crypts cells destroyed in the colon are observed.BaP = benzo(a)pyrene.
|
Expression of IL-6 and TNF-α in serum
Table 2
Expression of IL-6 and TNF-α in serum of mice in each group

| Groups | IL-6, pg/mL | TNF-α, pg/mL |
|---|---|---|
| Control | 3.24 ± 0.8 | 12.01 ± 1.6 |
| BaP 3 µg/kg | 12.54 ± 3.61a | 31.01 ± 4.89a |
| BaP 6 µg/kg | 26.54 ± 3.61a,b | 62.45 ± 6.22a,b |
IL-6, NF-κB, p53, SOD1, β-catenin, MUC1, and MUC2 in the colon
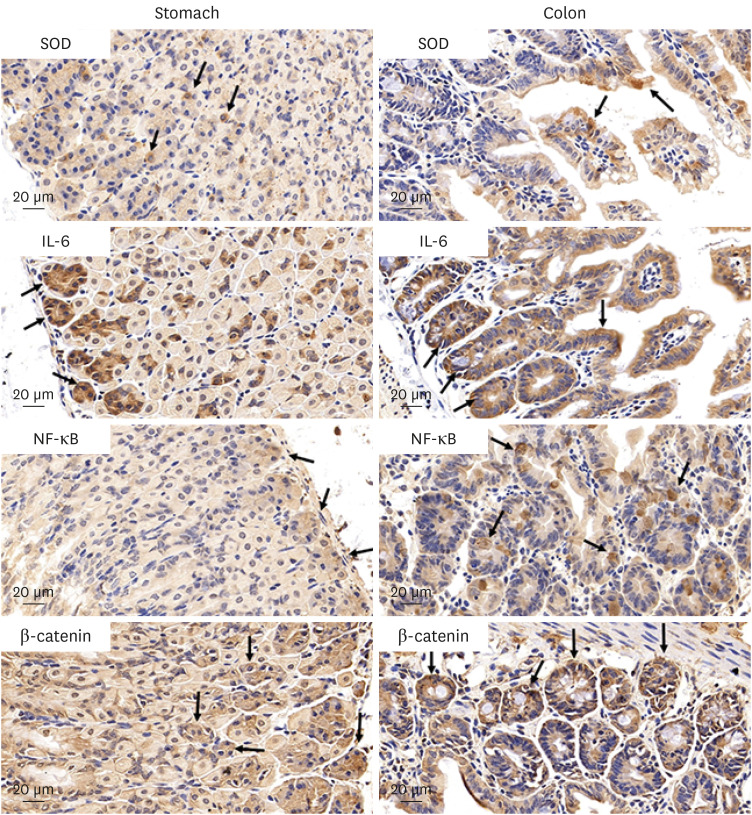 | Fig. 2Histologic expression of the antioxidant enzyme, the pro-inflammatory oncogenic cytokine, the tumor transcription factor, and the Wnt signaling cascade regulator of stomach and colon tissues are detected using immunohistochemical stains after daily oral intake of BaP for 60 days. SOD, IL-6, NF-κB, and β-catenin are expressed (yellowish-brown color) in the stomach and colon of mice, respectively.BaP = benzo(a)pyrene, SOD = superoxide dismutase, IL = interleukin, NF = nuclear factor.
|
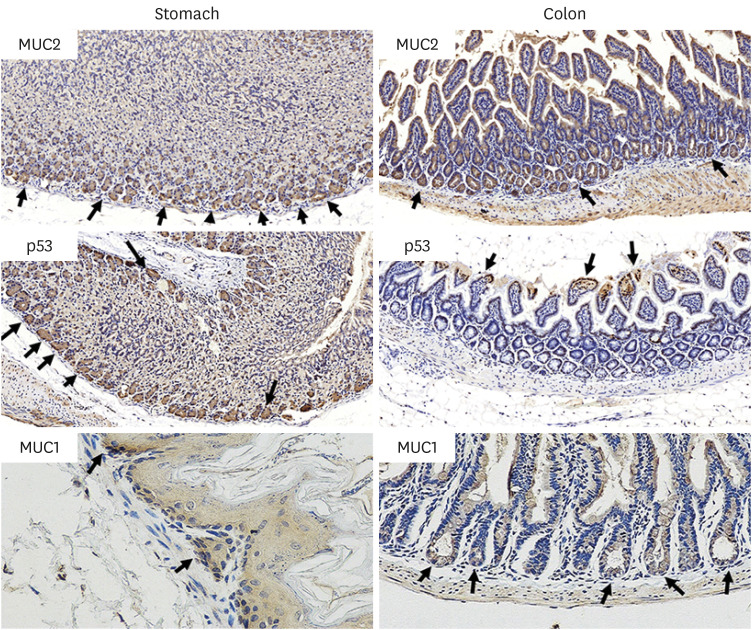 | Fig. 3Histologic expression of the indicator cancer markers: MUC2, p53, and MUC1 in stomach and colon tissues, are detected by using immunohistochemical stains after daily oral intake of BaP for 60 days. MUC2, p53, and MUC1 in the stomach and colon of mice, respectively.MUC = mucin glycoprotein, BaP = benzo(a)pyrene.
|
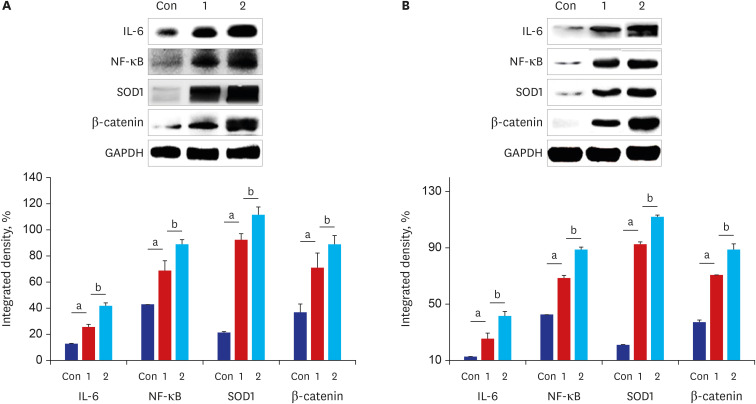 | Fig. 4Expression levels of the antioxidant enzyme, the pro-inflammatory oncogenic cytokine, the tumor transcription factor, and the Wnt signaling cascade regulator in the stomach and the colon tissue of mice are induced (n = 8). The stomach and the colon tissue were collected from mice after daily oral intake of BaP for 60 days. (A) Performing western blot analysis of IL-6, NF-κB, SOD1, β-catenin using antibodies and this graph illustrating the changes in the levels of IL-6, NF-κB, SOD1, β-catenin in the stomach were evaluated and compared to oral intake of controls vehicle (Con) and 3 µg/kg BaP (1) or 6 µg/kg BaP (2). (B) Performing western blot analysis of IL-6, NF-κB, SOD1, β-catenin in the colon tissues, and these graphs illustrating the changes in the levels of those were evaluated and compared. The densitometric values were normalized to levels of GAPDH expressed in each group. All data expressed the mean ± standard deviation of eight mice in each group.
aCompared to the control mice, the difference was statistically significant (P < 0.05); bCompared to mice between oral intake of 3 µg/kg BaP and 6 µg/kg BaP, the difference was statistically significant (P <0.05).
BaP = benzo(a)pyrene, IL = interleukin, NF = nuclear factor, SOD = superoxide dismutase, GAPDH = glyceraldehyde 3-phosphate dehydrogenase.
|
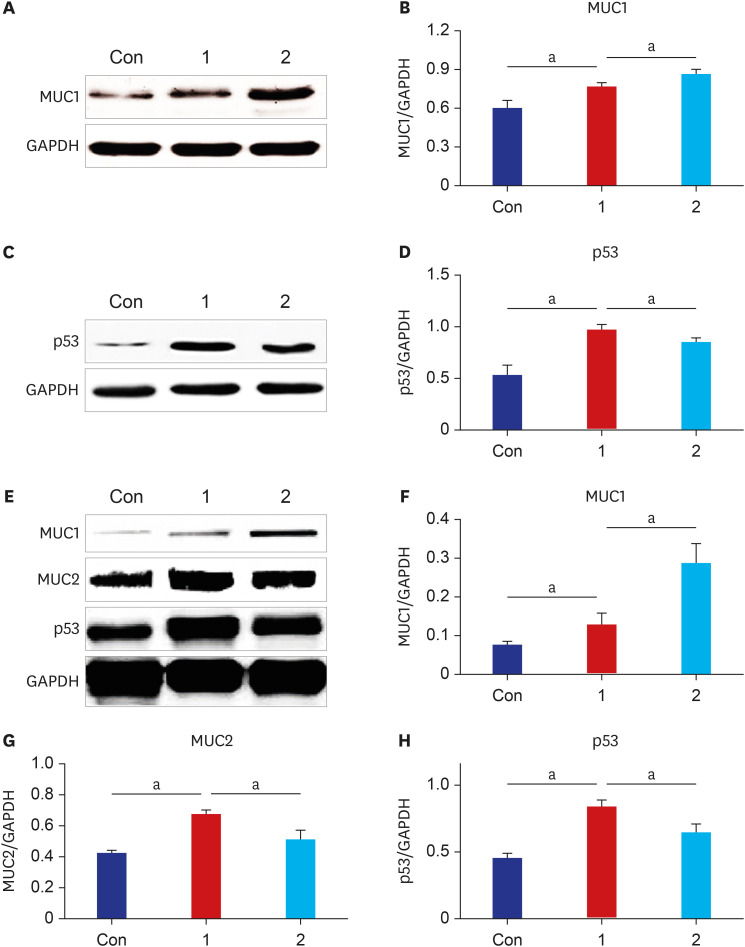 | Fig. 5Expression levels of the indicator cancer markers: MUC1, p53, and MUC2 in the stomach and the colon tissue of mice are induced (n = 8). The stomach and the colon tissue were collected from mice after daily oral intake of BaP for 60 days. (A) Performing western blot analysis of MUC1 using antibodies. (B) These graphs illustrate the changes in the levels of MUC1 in the stomach of each group; (C) Performing western blot analysis of p53 using antibodies, and (D) these graphs illustrate the changes in the levels of p53 in the stomach were evaluated and compared to oral intake of controls vehicle (Con) and 3 µg/kg BaP (1) or 6 µg/kg BaP (2). (E) Performing western blot analysis of MUC1, MUC2, and p53 in the colon tissues. (F, G, H) The graphs illustrating the changes in MUC1, MUC2, and p53 levels were evaluated and compared. The densitometric values were normalized to levels of GAPDH expressed in each group. All data expressed the mean ± standard deviation of eight mice in each group.MUC = mucin glycoprotein, BaP = benzo(a)pyrene, GAPDH = glyceraldehyde 3-phosphate dehydrogenase.
aThe difference was statistically significant (P < 0.05).
|




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download