Abstract
Purpose
Increasing evidence has shown an association of surgical technique, particularly anastomotic configuration, with postoperative recurrence of CD. This pilot study aimed to evaluate short-term outcomes of isoperistaltic side-to-side anastomosis (ISSA) employed on Crohn disease (CD) patients.
Methods
Data were retrieved from a prospectively maintained database. Postoperatively, all patients were followed up with close endoscopic (ileocolonoscopy) surveillance.
Results
From January 2017 to May 2021, 30 patients diagnosed with CD who underwent ISSA were compared with 45 CD patients who underwent antiperistaltic side-to-side anastomosis (ASSA). The 2 groups were comparable in baseline demographics and clinical characteristics. No significant differences were observed between groups regarding postoperative safety issues, including anastomotic leak, abdominal/pelvic abscess, length of hospital stay, readmission rate within 30 days, etc. At postoperative 24th month, reduced endoscopic recurrence was observed in the ISSA group compared with that in the ASSA group (18 of 24, 75.0%, vs. 36 of 38, 94.7%; P = 0.024). Regarding surgical recurrence, there was 0% in the ISSA group vs. 4.4% (2 of 45) in the ASSA group (P = 0.510).
Conclusion
In this study, we aimed to explore the influence of ISSA on postoperative recurrence in CD patients, and the preliminary results show that ISSA was technically safe and feasible, and appears to be effective in reducing postoperative recurrence in CD patients. However, our conclusion was underpowered due to small sample size and inadequate follow-up. We proposed ISSA be considered as another alternative option in the toolbox of inflammatory bowel disease surgeons when performing anastomosis on CD patients.
Despite advances in medical treatment in recent decades, surgery remains unavoidable in certain aspects of Crohn disease (CD) patients [1]. Recently, more and more studies have revealed that postoperative recurrence is associated with details of surgical techniques. Particularly, given the fact that most recurrences appeared in the perianastomotic or anastomotic sites [2], the configuration of anastomosis (e.g., side-to-side vs. end-to-end, manual vs. mechanical, and isoperistaltic vs. antiperistaltic) has gained more and more attention. A great number of evidences have shown that side-to-side anastomosis tended to reduce postoperative recurrence without more adverse complications [3], and it is widely accepted that a larger caliber of anastomotic lumen contributed to the lower rate of recurrence [4]. The most typical example is a recent novel anastomotic technique that is called Kono-S anastomosis [5]. Clinical studies adopting Kono-S anastomosis, not only from Japan but also from the United States [6] and European countries [7] are encouraging, as it further reduced recurrence as compared to the side-to-side anastomosis (conventionally called functional end-to-end anastomosis). Functional peristalsis, which led to decrease in fecal stasis, was assumed to play a role [2].
In recent years, we have explored the value of another type of anastomosis configuration—isoperistaltic side-to-side anastomosis (ISSA) while performing surgery for CD. As a matter of fact, ISSA is not novel; previous studies involving non-CD patients [89101112] or involving CD patients [13] had utilized the same or similar techniques. Results from all these studies have shown that the technique is safe and feasible regarding short-term safety outcomes. Furthermore, given the reason that ISSA is in isoperistaltic configuration, which is theoretically more physiological than antiperistaltic side-to-side anastomosis (ASSA), we speculated that ISSA, like Kono-S anastomosis, may also possess potential benefits (e.g., large anastomotic lumen, functional peristalsis) in reducing postoperative CD recurrence. Therefore, we conducted this preliminary, retrospective study to evaluate the outcomes of ISSA during surgery for CD patients, mainly focusing on the impact of anastomosis configuration on postoperative recurrence by comparing it with the conventional anastomosis group operated on by the same surgical team.
The study protocol was approved by the Institutional Review Board of Zhongnan Hospital of Wuhan University (No. 2017073). All methods were performed in accordance with the Declaration of Helsinki. All participants gave their signed informed consent prior to inclusion.
From January 2017 to May 2021, patients diagnosed with CD (according to the guideline criteria with comprehensive assessment of clinical, radiologic, endoscopic, and histopathologic findings) and underwent operation with bowel resection and anastomosis (resection of diseased segment of small bowel followed by anastomosis in ileum or jejunum; ileocaecal resection followed by ileocolic anastomosis; resection of diseased segment of large bowel followed by colocolic/colorectal anastomosis; subtotal/total colectomy followed by ileorectal anastomosis; also including reversal of stoma) at the Inflammatory Bowel Disease Center in Zhongnan Hospital of Wuhan University, were included in this single-institutional, retrospective study. Only patients who received single intestinal resection and anastomosis were included, while patients who received multiple intestinal resections (≥2) followed by multiple intestinal anastomoses were excluded (n = 12). Furthermore, patients with follow-up of less than 6 months (n = 10) and patients with incomplete/missing data (n = 8) were excluded from the study (as shown in flow diagram of Fig. 1). The decision to perform ISSA was made intraoperatively by the surgeon according to certain selection criteria including cases with more severe edema and fibrosis of intestinal wall, more severe abdominal adhesions and less extent of intestinal mobilization, etc. All data were collected and retrieved from a prospectively well-maintained database.
Postoperatively, all patients were closely followed up, medical prophylaxis treatments were given to patients by a specialized inflammatory bowel disease (IBD) gastroenterologist following guidelines of The European Crohn’s and Colitis Organization (ECCO) [14]. Total endoscopy surveillance (if not assessable by ileocolonoscopy, capsule endoscopy was utilized otherwise) was recommended every 6 months in the first postoperative year (POY 1), and then annually. Particularly, for patients with ileocolic anastomosis, a well-developed Rutgeerts’ scoring system [15] was employed to evaluate endoscopic recurrence, and a Rutgeerts’ score of ≥i2 is defined as endoscopic recurrence. As for patients with other than ileocolic anastomosis (e.g., ileal/jejunal, colocolic/colorectal), endoscopic recurrence was defined with endoscopic finding of diffuse inflamed mucosa with ulcers, nodules, and/or narrowing (diagnosis established by the same specialized, experienced gastroenterologist). Reoperation for any perianastomotic recurrence is defined as surgical recurrence, whereas recurrence in other separate intestinal segments or sites was not considered surgical recurrence and was excluded.
All patients were operated on in an open procedure or using laparoscopic assistance (requiring a small incision for specimen extraction and construction of extracorporeal anastomosis).
The diseased intestine segment was transected perpendicularly using an open linear stapler (NTLC75, Ethicon, Raritan, NJ, USA). Care should be taken to preserve as much healthy mesentery as possible to maintain adequate vascularization and innervation of the residual intestine. The 2 staple lines were reinforced and buried with interrupting sutures using 3-0 Vicryl (Ethicon). The proximal and distal limbs were then approximated and overlapped side-to-side in an isoperistaltic orientation. Antimesenteric enterotomies were then created (approximately 8 cm proximal to the stapler line of the proximal limb; 1–2 cm proximal to the stapler line of the distal limb) to allow the entrance of the 2 jaws of a linear stapler (Figs. 2, 3). Side-to-side anastomosis was performed. Lastly, the common enterotomy was closed using a 2-layer, running 3-0 Vicryl suture. The mesenteric defect was closed if feasible.
ASSA, also called functional end-to-end anastomosis, is a more widely used surgical technique during colorectal operations including surgery for CD. When performing ASSA, the proximal and distal limbs were approximated on in a side-to-side antiperistaltic orientation, then a linear stapler (NTLC75) was inserted into both limbs, and then anastomosis was performed. The common enterotomy was transected and closed with another rod of a stapler cartilage. The stapler line was usually reinforced with interrupted suture using 3-0 Vicryls.
Categorical variables were presented as frequency and percentages and continuous variables were presented using mean ± standard deviation if they were normally distributed; otherwise, as median (range). Continuous variables were compared by independent group t-tests when the data were normally distributed; otherwise, using Mann-Whitney test. Categorical variables were compared using the chi-square test; otherwise, Fisher exact test if the sample size was limited. Multivariable analysis was performed using the stepwise backward method (Wald), which included variables with a P-value of <0.1 on univariable analysis. A P-value of <0.05 was considered statistically significant.
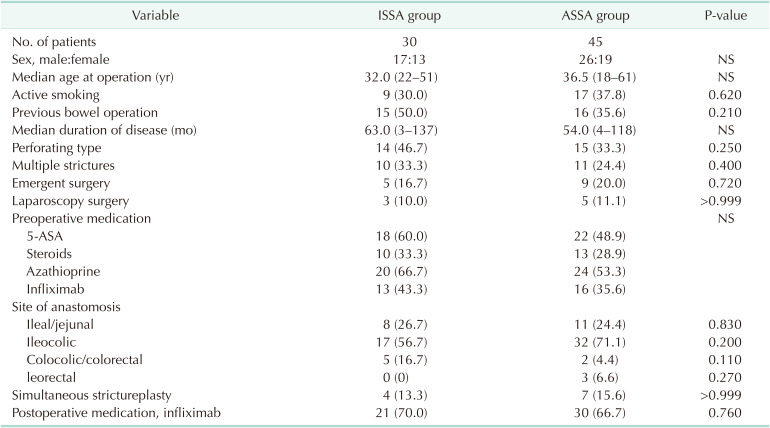
During the study period, a total of 30 CD patients who underwent ISSA were included. Forty-five CD patients who underwent ASSA were included and served as comparator group. The baseline demographics and clinical characteristics of these 2 groups (Table 1) are comparable (P ≥ 0.05), including sex, age, disease duration, smoking status, history of bowel operation, type and emergency of operation, disease behavior (perforating type), and preoperative medication. Regarding operation details, ileocaecal resection followed by ileocolic anastomosis accounts for the majority of cases in the 2 cohorts (56.7% in ISSA vs. 71.1% in ASSA, P = 0.200).
There appeared to be more colocolic/colorectal anastomosis in the ISSA group than in the ASSA group after segmental colectomy (16.7% vs. 4.4%, P = 0.110). Three patients (6.7%) with multiple or extensive involved colonic segments received subtotal/total colectomy followed by ileorectal anastomosis in the ASSA group; as opposed to nil in the ISSA group (P = 0.270). Postoperatively, medical prophylaxis treatments with anti-tumor necrosis factor alpha (infliximab) therapy were comparable between the 2 groups.
As shown in Table 2, ISSA seemed to be more time-consuming, with a mean time of 152 minutes vs. 141 minutes in ASSA, although it was not statistically significant (P = 0.090). Regarding postoperative outcomes, e.g., anastomosis leak, abdominal/pelvic abscess, ileus, no significant difference was observed between groups. Anastomotic leak (AL) was 1 (3.3%) vs. 4 (8.9%) in ASSA and ISSA, respectively. Though seemingly reduced in the ASSA group, P-value was not significant. As for other postoperative morbidities including abdominal/pelvic abscess, ileus, both groups were comparable. Length of hospital stay and rates of readmission, reoperation, and mortality within 30 days were also not significantly different between the 2 groups. Particularly, 2 patients in the ASSA groups required reoperation because of severe intestinal obstruction and AL after failure of conservative treatments.
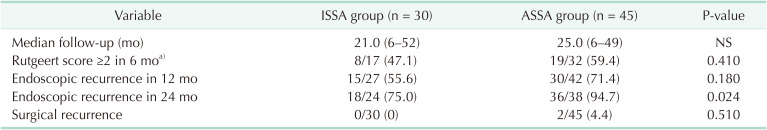
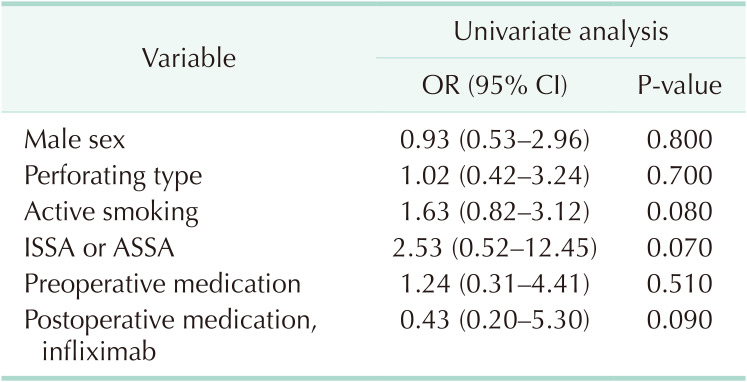
As shown in Table 3, after a median follow-up of 21 months (range, 6–52 months) in the ISSA group and 25 months (range, 6–49 months) in the ASSA group, endoscopic recurrence (Rutgeerts score of ≥i2 in patients of ileocolic anastomosis) was observed in 8 of 17 (47.1%) and 19 of 32 patients (59.4%) in the ISSA and ASSA groups, respectively, in the 6th postoperative month (the POM 6). In the POM 12, endoscopic recurrence (including patients of all anastomotic sites) was observed in 15 of 27 (55.6%) and 30 of 42 patients (71.4%) in the ISSA and ASSA groups (P = 0.180), respectively. While in the POM 24, it was observed that endoscopic recurrence (including patients of all anastomotic sites) was significantly reduced in the ISSA group (18 of 24, 75.0%) as compared with that (36 of 38, 94.7%) in the ASSA group (P = 0.024). Until the latest follow-up, surgical recurrence occurred in 2 patients in the ASSA group: one case was a 33-year-old male who experienced recurrence in the anastomotic sites in the POM 20 due to stenosis at anastomosis; another case was a 48-year-old female who refused postoperative medical treatment, and reoperation was performed in the POM 43 due to fistula around the anastomosis. Though no surgical recurrence occurred in the ISSA group, the difference between the 2 groups was not significant (P = 0.510). The multivariable analysis aiming to analyze factors associated with endoscopic recurrence at the POM 12 was performed using the following variables: male sex (yes/no), perforating type of CD behavior (yes/no), active smoking (yes/no), ISSA vs. ASSA, preoperative medication (yes/no), and postoperative medication (infliximab) (yes/no). However, no variable was significantly associated with the reduced risk of endoscopic recurrence (Table 4). Due to the limited observed event, we could not find a significantly lower probability of surgical recurrence by Kaplan-Meier analysis, either.
ISSA was first reported in 2005 by Tewari and Shukla [12] when performing ileocolic anastomosis after right colectomy. Since then, reports from many groups [891011] utilizing similar techniques, or its analog, have confirmed the safety and feasibility of this anastomotic technique. In fact, when constructing intracorporeal ileocolic anastomosis after right colectomy, ISSA is commonly used [1617], and many comparative clinical trials have also proved the safety of an intracorporeal ISSA [1618]. However, none of the above studies involved CD patients. Recently, a multicentric study from the Italian Society of Colorectal Surgery reported that 175 CD patients (41%) had undergone ISSA and showed there was no difference in postoperative morbidity between ISSA and ASSA [13]. However, this study only focused on short-term safety profiles associated with anastomotic technique and configuration, without paying any attention to the impact of anastomosis configuration on postoperative recurrence. As far as we know, our study is the first study to specifically explore to influence of ISSA on postoperative recurrence in CD patients.
Undoubtedly, postoperative recurrence remains one of the biggest unsolved issues for CD [1]. Although certain risk factors have been identified to be associated with postoperative recurrence, some of them (e.g., perforating disease, previous resection, and extensive disease) are unmodifiable and unpreventable. Recently, growing interest has been garnered toward the new concept of surgical prophylaxis for CD [26]. One of the most remarkable examples is Kono-S anastomosis. In a recent meta-analysis that enrolled 9 studies (896 patients), Kono-S anastomosis was related to a lower incidence of endoscopic and surgical recurrence [19]. Likewise, in our study, endoscopic recurrences were also reduced at the POM 6 and POM 12 in the ISSA group; however, not until the POM 24 can we observe a statistically significant difference (75.0% in ISSA vs. 94.7% in ASSA, P = 0.024). Our results were consistent with the first report of Kono et al. [5], in which endoscopic recurrence of Kono-S and its comparator were not significantly different until the POY 5. In a recent randomized clinical trial of Luglio et al. [7], Kono-S anastomosis was significantly lower in endoscopic recurrence both in the POM 6 and 18 assessments. Nevertheless, short-term endoscopic recurrence may predict and translate into subsequent symptomatic recurrence [1520]. Surgical recurrence, was both 0% in the Kono-S group in the above 2 studies [57], whereas 4.6% (the POY 2) [7] and 15% (the POY 5) [5] in the comparator group. Our results were approximated to them: no surgical recurrence in the ISSA group while 4.4% occurred in the comparator group. Although no firm conclusion of reducing postoperative recurrence in CD patients by ISSA could be drawn from the present study, we believed the limited sample size and inadequate follow-up were to blame. As time goes by, we believe a statistically significant difference in surgical recurrence would eventually be gained at a certain time node.
In recent years, the mechanisms underlying the influence of anastomotic construction on postoperative CD recurrence have been intensely studied. Perianastomotic factors, including localized ischemia and denervation, local cytokines production, and changes in the local microbiome, which partially result from fecal stasis, increased transit time, and impaired motility are believed to be related to postoperative recurrence [2]. Using an animal model, Kono and Fichera [2] reported reduced transit time in Kono-S group as compared to the side-to-side configuration group (data not published). Technically, ISSA shows similar benefits to Kono-S anastomosis from these perspectives. First, both ISSA and Kono-S have wide anastomotic lumen caliber and are in isoperistalsis configuration, which together contribute to the functional peristalsis. Interestingly, the term “functional end-to-end anastomosis” [5] was used both in ASSA and Kono-S anastomosis [21]. As a matter of fact, ASSA is not “end-to-end” but “side-to-side” in configuration, and experimental studies [222324] have also proved ASSA was less functional than end-to-end anastomosis regarding intestinal motility and bacterial growth. From this viewpoint, Kono-S anastomosis might better deserve the naming of “functional end-to-end anastomosis.” Secondly, intervening mesentery in both ISSA and Kono-S can be divided close to the bowel, resulting in better preservation of vascularization and innervation [5]. Thirdly, the mesentery, which is believed to play an important role in CD recurrence [25], is well isolated from the anastomotic lumen by the central “supporting column” in Kono-S anastomosis. Although the mesentery in ISSA seems to be not so perfectly excluded from anastomotic lumen as Kono-S. However, the role of the mesentery remains controversial [26] and 2 contradictory strategies, i.e., mesenteric preservation in Kono-S anastomosis [5] and extended mesenteric resection [25] seem both to have reduced recurrence, which is currently under fierce debate [192728]. Furthermore, how functional peristalsis (defined as increased intestinal motility, lessened fecal stasis, and inhibited bacterial overgrowth) can eventually translate into reduced postoperative CD recurrence remains to be answered by sufficient further basic and clinical studies.
Compared with the Italian multicentric study [13], our study applied ISSA not only on ileocolic anastomosis following ileocaecal resection in CD patients, but also on other anatomic sites, i.e., ileal/jejunal, colocolic/colorectal, and ileorectal. Matsuda et al. [11] demonstrated that the most remarkable advantage of the isoperistaltic anastomosis was that it required less intestinal mobilization to allow a tension-free anastomose, which could theoretically save as much as a 6-cm length of mobilized bowel [11]. Consequently, ISSA is expected to be a more suitable indication for colocolic anastomosis [11] and colorectal anastomosis [910], in which mobilization of bowel is usually more demanding. That probably explains the reason more ISSA was performed in colocolic and colorectal anastomosis in our study, as we considered ISSA technically more preferable. When operating on CD patients, inflammation, adhesion, and fibrosis are usually more severe, making it more difficult to perform mobilization and anastomosis; hence, ISSA might be more helpful in these harsh situations and consequently reduce postoperative complications. In our study, the AL rate in the ISSA group was relatively lower than that of the ASSA group (3.3% vs. 8.9%), although not reaching a statistical significance (P = 0.340). Undoubtedly, tension is one of the most critical factors for intestinal anastomosis [293031]. Theoretically, if anastomosis tension can be decomposed transversely and longitudinally, additional transverse tension is exerted on ASSA. Nevertheless, the pathogenesis mechanism of gastrointestinal anastomosis is complex and remains unknown [29]. Furthermore, intestinal anastomosis in CD patients who are usually accompanied by risk factors such as malnutrition, low albumin, intestinal obstruction, and steroid use are at higher risk of the leak [32]. Hence, ISSA, with the aforementioned theoretical advantages, is assumed to bring more benefits to intestinal anastomosis in CD patients, especially in cases with more severity of abdominal adhesions, edema, and fibrosis of intestinal wall.
To sum up, based on our experience and previously reported literatures, the advantages of ISSA included good safety profiles, assumed benefits to intestinal anastomosis, and potential prophylaxis of postoperative CD recurrence. The disadvantage of ISSA is that it required a hand-sewn suture to close the common stoma, which is relatively more time-consuming; though on the other hand, easy to perform even in inexperienced hands. We acknowledge that several inherent limitations existed in our study. First and foremost, as the primary outcome of the study, we could not provide a very firm and powerful conclusion that ISSA can reduce postoperative CD recurrence. We believe it is not difficult to reach a statistical significance with cases accumulated and follow-up extended. However, even if this is realized, like Kono-S anastomosis, definitively proving the concept of anastomotic configuration on surgical prophylaxis of CD remains distant. Nonetheless, our study has shown that ISSA may serve as an alternative option in the toolbox of IBD surgeons when performing anastomosis on CD patients, given its assumed benefits to intestinal anastomosis. Secondly, this study is in retrospective nature and cannot remove all selection or recall bias. Thirdly, the sample size is relatively small, and the results of long-term follow-up are not provided. However, the primary purpose of this pilot study, which is to report short-term outcomes, to some extent is realized. To better prove the value of ISSA, direct comparative studies with expanded cases and extended follow-up among ISSA, ASSA with Kono-S anastomosis related to postoperative CD recurrence are warranted in the future.
Collectively, this preliminary study reports short-term results of employing ISSA on CD patients. We showed that ISSA was technically safe and feasible, and may potentially reduce postoperative recurrence in CD patients. However, due to the limitations of small sample size and lack of long-term follow-up, our conclusion was underwhelming. Based on many theoretical advantages and benefits, we proposed that ISSA be considered as an additional alternative option in the toolbox of IBD surgeons when performing anastomosis on CD patients. Further studies must be carried out and these results are eagerly awaited in the future.
Notes
References
1. De Cruz P, Kamm MA, Hamilton AL, Ritchie KJ, Krejany EO, Gorelik A, et al. Crohn’s disease management after intestinal resection: a randomised trial. Lancet. 2015; 385:1406–1417. PMID: 25542620.

2. Kono T, Fichera A. Surgical prophylaxis of Crohn disease recurrence: “light at the end of the tunnel”. Ann Surg. 2020; 272:218–219. PMID: 32675484.
3. Feng JS, Li JY, Yang Z, Chen XY, Mo JJ, Li SH. Stapled side-to-side anastomosis might be benefit in intestinal resection for Crohn’s disease: a systematic review and network meta-analysis. Medicine (Baltimore). 2018; 97:e0315. PMID: 29642162.
4. Adamina M, Bonovas S, Raine T, Spinelli A, Warusavitarne J, Armuzzi A, et al. ECCO guidelines on therapeutics in Crohn’s disease: surgical treatment. J Crohns Colitis. 2020; 14:155–168. PMID: 31742338.

5. Kono T, Ashida T, Ebisawa Y, Chisato N, Okamoto K, Katsuno H, et al. A new antimesenteric functional end-to-end handsewn anastomosis: surgical prevention of anastomotic recurrence in Crohn’s disease. Dis Colon Rectum. 2011; 54:586–592. PMID: 21471760.

6. Kono T, Fichera A, Maeda K, Sakai Y, Ohge H, Krane M, et al. Kono-S anastomosis for surgical prophylaxis of anastomotic recurrence in Crohn’s disease: an international multicenter study. J Gastrointest Surg. 2016; 20:783–790. PMID: 26696531.

7. Luglio G, Rispo A, Imperatore N, Giglio MC, Amendola A, Tropeano FP, et al. Surgical prevention of anastomotic recurrence by excluding mesentery in Crohn’s disease: the SuPREMe-CD Study: a randomized clinical trial. Ann Surg. 2020; 272:210–217. PMID: 32675483.

8. Ibáñez N, Abrisqueta J, Luján J, Hernández Q, Rufete MD, Parrilla P. Isoperistaltic versus antiperistaltic ileocolic anastomosis: does it really matter?: results from a randomised clinical trial (ISOVANTI). Surg Endosc. 2019; 33:2850–2857. PMID: 30426254.

9. Kawahara H, Hirai K, Watanabe K, Kashiwagi H, Yamazaki Y, Yanaga K. Sliding functional end-to-end anastomosis for colorectal surgery. Int Surg. 2007; 92:34–36. PMID: 17390913.
10. Kawahara H, Kobayashi T, Watanabe K, Shinoda T, Kashiwagi H, Yanaga K. Colorectal stapling anastomosis without transanal procedure for anterior reseciton. Hepatogastroenterology. 2009; 56:352–354. PMID: 19579597.
11. Matsuda A, Miyashita M, Matsumoto S, Sakurazawa N, Takahashi G, Yamada M, et al. Isoperistaltic versus antiperistaltic stapled side-to-side anastomosis for colon cancer surgery: a randomized controlled trial. J Surg Res. 2015; 196:107–112. PMID: 25818976.

12. Tewari M, Shukla HS. Right colectomy with isoperistaltic side-to-side stapled ileocolic anastomosis. J Surg Oncol. 2005; 89:99–101. PMID: 15660366.

13. Celentano V, Pellino G, Spinelli A, Selvaggi F, Celentano V, et al. SICCR Current status of Crohn’s disease surgery collaborative. Anastomosis configuration and technique following ileocaecal resection for Crohn’s disease: a multicentre study. Updates Surg. 2021; 73:149–156. PMID: 33409848.

14. Torres J, Bonovas S, Doherty G, Kucharzik T, Gisbert JP, Raine T, et al. ECCO guidelines on therapeutics in Crohn’s disease: medical treatment. J Crohns Colitis. 2020; 14:4–22. PMID: 31711158.

15. Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the postoperative course of Crohn’s disease. Gastroenterology. 1990; 99:956–963. PMID: 2394349.

16. Allaix ME, Degiuli M, Bonino MA, Arezzo A, Mistrangelo M, Passera R, et al. Intracorporeal or extracorporeal ileocolic anastomosis after laparoscopic right colectomy: a double-blinded randomized controlled trial. Ann Surg. 2019; 270:762–767. PMID: 31592811.

17. Hanna MH, Hwang GS, Phelan MJ, Bui TL, Carmichael JC, Mills SD, et al. Laparoscopic right hemicolectomy: short- and long-term outcomes of intracorporeal versus extracorporeal anastomosis. Surg Endosc. 2016; 30:3933–3942. PMID: 26715015.

18. Bollo J, Turrado V, Rabal A, Carrillo E, Gich I, Martinez MC, et al. Randomized clinical trial of intracorporeal versus extracorporeal anastomosis in laparoscopic right colectomy (IEA trial). Br J Surg. 2020; 107:364–372. PMID: 31846067.

19. Alshantti A, Hind D, Hancock L, Brown SR. The role of Kono-S anastomosis and mesenteric resection in reducing recurrence after surgery for Crohn’s disease: a systematic review. Colorectal Dis. 2021; 23:7–17. PMID: 32418300.

20. Allez M, Lémann M. Role of endoscopy in predicting the disease course in inflammatory bowel disease. World J Gastroenterol. 2010; 16:2626–2632. PMID: 20518084.

21. Kano M, Hanari N, Gunji H, Hayano K, Hayashi H, Matsubara H. Is “functional end-to-end anastomosis” really functional?: a review of the literature on stapled anastomosis using linear staplers. Surg Today. 2017; 47:1–7. PMID: 26988855.

22. Arnold JH, Alevizatos CA, Cox SE, Richards WO. Propagation of small bowel migrating motor complex activity fronts varies with anastomosis type. J Surg Res. 1991; 51:506–511. PMID: 1943088.

23. Hocking MP, Carlson RG, Courington KR, Bland KI. Altered motility and bacterial flora after functional end-to-end anastomosis. Surgery. 1990; 108:384–392. PMID: 2382231.
24. Toyomasu Y, Mochiki E, Ando H, Yanai M, Ogata K, Tabe Y, et al. Comparison of postoperative motility in hand-sewn end-to-end anastomosis and functional end-to-end anastomosis: an experimental study in conscious dogs. Dig Dis Sci. 2010; 55:2489–2497. PMID: 19915979.

25. Coffey CJ, Kiernan MG, Sahebally SM, Jarrar A, Burke JP, Kiely PA, et al. Inclusion of the mesentery in ileocolic resection for Crohn’s disease is associated with reduced surgical recurrence. J Crohns Colitis. 2018; 12:1139–1150. PMID: 29309546.

26. Coffey JC, O’Leary DP, Kiernan MG, Faul P. The mesentery in Crohn’s disease: friend or foe? Curr Opin Gastroenterol. 2016; 32:267–273. PMID: 27115218.
27. Luglio G, Imperatore N, Tropeano FP, Rispo A. Response to the comment on “Surgical prevention of anastomotic recurrence by excluding mesentery in Crohn’s disease: the SuPREMe-CD Study: a randomized clinical trial”. Ann Surg. 2021; 274:e738–e739. PMID: 32976274.
28. Zhou W. Comment on “Surgical prevention of anastomotic recurrence by excluding mesenter y in Crohn’s disease: the SuPREMe-CD Study: a randomized clinical trial”. Ann Surg. 2021; 274:e737–e738. PMID: 32773634.
29. Chadi SA, Fingerhut A, Berho M, DeMeester SR, Fleshman JW, Hyman NH, et al. Emerging trends in the etiology, prevention, and treatment of gastrointestinal anastomotic leakage. J Gastrointest Surg. 2016; 20:2035–2051. PMID: 27638764.

30. Cho SH, Lee IK, Lee YS, Kim MK. The usefulness of transanal tube for reducing anastomotic leak in mid rectal cancer: compared to diverting stoma. Ann Surg Treat Res. 2021; 100:100–108. PMID: 33585354.

31. Nam SH. A novel and simple method using a transanal intestinal long tube for protecting intestinal anastomosis and decompressing the small bowel. Ann Surg Treat Res. 2017; 93:137–142. PMID: 28932729.

32. Huang W, Tang Y, Nong L, Sun Y. Risk factors for postoperative intra-abdominal septic complications after surgery in Crohn’s disease: a meta-analysis of observational studies. J Crohns Colitis. 2015; 9:293–301. PMID: 25572276.

Fig. 1
Flow diagram showing the process of included cases and excluded cases. CD, Crohn disease; ISSA, isoperistaltic side-to-side anastomosis; ASSA, antiperistaltic side-to-side anastomosis.

Fig. 2
Diagrammatic representation of isoperistaltic side-to-side anastomosis. Antimesenteric enterotomies were created to allow the entrance of the 2 jaws of a linear stapler.
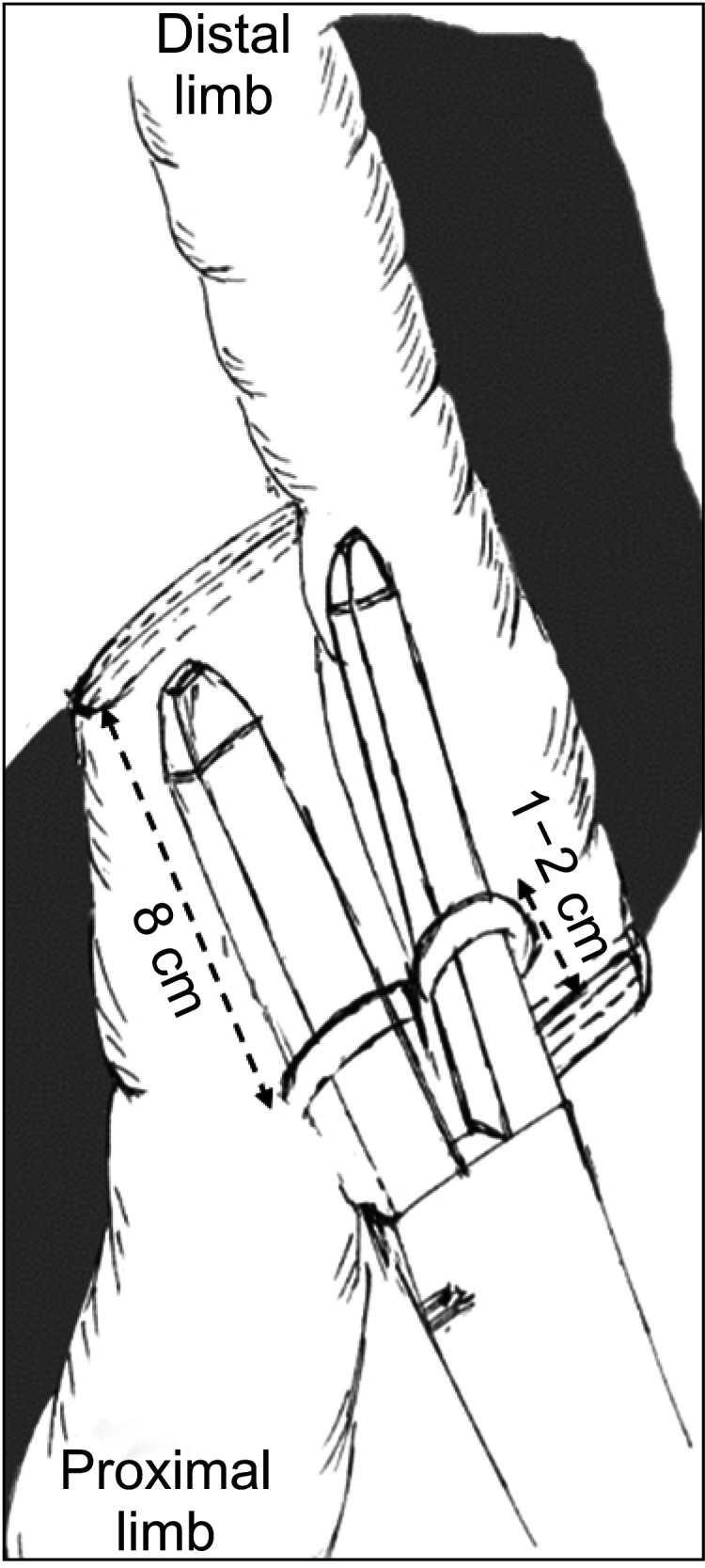
Fig. 3
Surgery pictures demonstrating isoperistaltic side-to-side anastomosis. (A) An open linear stapler (NTLC75, Ethicon, Raritan, NJ, USA) was used to perform isoperistaltic side-to-side anastomosis. The 2 jaws of linear stapler were inserted into the antimesenteric enterotomies. After firing one stapler load, a side-to-side anastomosis was constructed. The common enterotomy was then closed using a 2-layer, running 3-0 Vicryl suture (Ethicon). (B) Surgical view of a completed isoperistaltic side-to-side anastomosis.
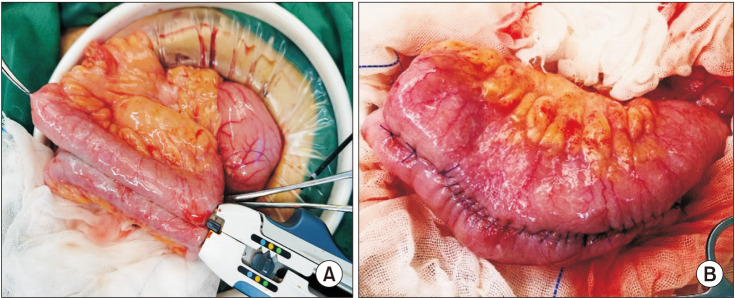
Table 2
Operative variables and postoperative outcomes comparing ISSA with ASSA
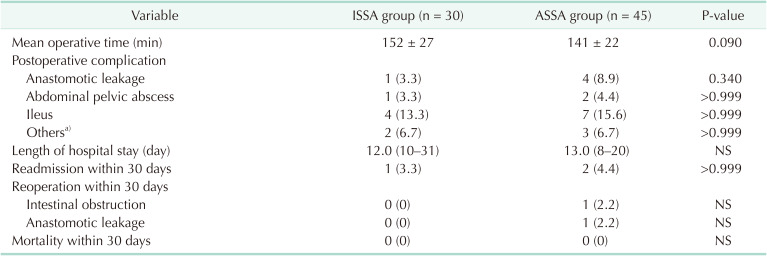
Values are presented as mean ± standard deviation, number (%), or median (range).
ISSA, isoperistaltic side-to-side anastomosis; ASSA, antiperistaltic side-to-side anastomosis; NS, no significant differences.
a)Other minor complications include incisional infection, urinary retention, and pneumonia.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download