Abstract
Purpose
The surgical success rate for primary hyperparathyroidism (PHPT) is currently 95%–98%. However, 3%–24% of patients show persistently elevated (Pe) parathyroid hormone (PTH) levels after parathyroidectomy (PTX). This single-center retrospective study aimed to compare the outcomes of patients with normal PTH and PePTH levels after successful PTX and to identify the factors associated with PePTH.
Methods
The normal group, defined as patients with normal serum calcium and PTH levels immediately after PTX, was compared with the PePTH group (patients with normal or low serum calcium and increased serum PTH levels up to 6 months postoperatively) to determine the causes of disease in the PePTH group.
Results
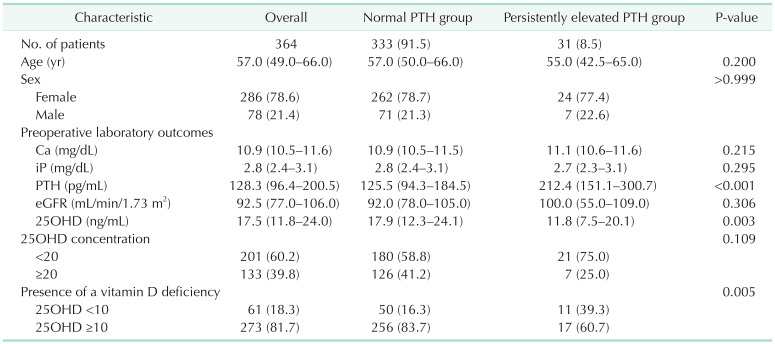
There were no significant differences in age, sex, or preoperative estimated glomerular filtration rate between the normal PTH group (333 of 364, 91.5%) and the PePTH group (31 of 364, 8.5%). However, there were significant differences in preoperative 25-hydroxyvitamin D (17.9 and 11.8 ng/mL, respectively; P = 0.003) and PTH levels (125.5 and 212.4 pg/mL, respectively; P < 0.001) between the 2 groups. Among the 31 cases of the PePTH group, 18 were attributed to vitamin D deficiency.
Conclusion
Preoperative vitamin D deficiency is a predictive factor for PePTH. Therefore, preoperative administration of vitamin D supplements may reduce the probability of postoperative disease persistence. Patients with temporary laboratory abnormalities within 6 months after successful PTX should be monitored, and appropriate vitamin D and calcium supplementation may reduce the effort and cost of various examinations or reoperations.
Go to : 
Primary hyperparathyroidism (PHPT) is defined as a calcium (Ca) metabolism disorder characterized by abnormally increased serum Ca and elevated parathyroid hormone (PTH) levels. PHPT is the most common cause of hypercalcemia, and most cases are accidentally detected through blood tests before symptoms appear in patients [12]. Patients who do not respond to treatment have an increased risk of renal, bone, and cardiovascular diseases [34].
Surgical treatment is the treatment of choice for PHPT. The purpose of the surgical treatment is to remove the hyperfunctioning gland. The conventional operation method comprises a thorough visual exploration of all 4 parathyroid glands and the resection of the hyperfunctioning gland. In recent years, the minimally invasive surgical approach has been the preferred option. The development and use of diagnostic imaging tools before surgery and ability to assess intraoperative PTH (IOPTH) during surgery have greatly contributed to minimally invasive surgery. The high success rate of minimally invasive surgery has been reported in several studies [56].
The primary surgical success rate of PHPT is reported to be 95%–98%; however, 3%–24% of the patients show persistently elevated PTH (PePTH) with normal or low Ca levels after surgery [678]. While some studies have suggested that this phenomenon is temporary and presents only within the first year after surgery, some studies consider it to be a precursor to recurrent or persistent hyperparathyroidism [91011]. Risk factors for this complication include old age, high preoperative PTH levels, osteoporosis, decreased renal function, vitamin D deficiency, larger adenomas, and postoperative hypocalcemia [3812].
The recognized definition of recurrent PHPT states that there is an increase in serum Ca and PTH levels preoperatively and at 6 months postoperatively [41213]. Causes include failure of localization of the pathologic gland during the first surgery, no radical excision, or improper resection due to the presence of multiple adenomas [14]. However, in many cases, there is an increase in PTH levels with normal or low Ca up to 6 months after the initial surgery, followed by remission thereafter.
In this study, patients who underwent PHPT surgery in a single center were observed for more than 6 months and retrospectively analyzed. This study aimed to compare and analyze the normal PTH and PePTH groups after surgery in patients with PHPT and to identify the factors associated with PePTH. The goal was to ensure that these surgeries were performed properly and to reduce the effort and cost of various examinations or reoperations, and to reduce patient discomfort by taking several factors into account. The primary endpoint was to determine the rate of PePTH after surgery, and the secondary endpoint was to analyze the causes of each uncured patient.
Go to : 
This study was conducted according to the Declaration of Helsinki (1964) and its protocol was approved by the Severance Hospital Institutional Review Board (No. 2020-2447-001). All patients provided written informed consent before surgery regarding the surgical methods and possible postoperative complications.
This was a retrospective study of patients who underwent parathyroidectomy (PTX) with PHPT at Severance Hospital between January 2010 and December 2019. During this period, 469 patients underwent PHPT surgery. We excluded 1 patient who experienced recurrence after a previous surgery and underwent a reoperation, 2 with familial isolated hyperparathyroidism (FIHPT), 30 with multiple endocrine neoplasias (MENs), 8 who were diagnosed with parathyroid carcinoma in postoperative pathology, and 56 who were lost to follow up within 6 months after surgery. In addition, 7 patients with recurrence and 1 with an elevated serum Ca level were excluded. Finally, 364 patients were included in the study.
Blood samples were obtained before surgery. Serum electrolytes, including Ca and inorganic phosphorous (iP), estimated glomerular filtration rate (eGFR), and PTH were routinely monitored. The normal ranges of Ca and PTH were defined as 8.5–10.5 mg/dL and 15–65 pg/mL, respectively. The serum 25-hydroxyvitamin D (25OHD) concentration was checked during preoperative routine examinations. Vitamin D deficiency was defined as a 25OHD level of less than 10 ng/mL. The patients’ clinicopathologic characteristics are shown in Table 1. All patients underwent neck ultrasonography and a 99m technetium-sestamibi scan to determine the location of the pathologic glands before surgery. In addition, when the lesions were difficult to locate using the sestamibi scan and neck ultrasonography, other investigative techniques were performed. These included 4-dimensional CT, methionine PET, 18F-fluorocholine PET imaging, or parathyroid venous sampling.
All patients underwent PTX under general anesthesia. The surgical methods included open PTX in 354 patients and robot-assisted transaxillary approach PTX in 10. In the preoperative evaluation, if the pathologic gland was localized, focused PTX was performed; otherwise, full exploration was performed.
At this center, IOPTH monitoring began in 2015 and of the 364 patients, 230 (63.2%) had their IOPTH levels checked. Baseline blood samples were collected before surgery and at 5 and 10 minutes after the parathyroid gland excision through the radial arterial line. If there was more than 1 pathological gland, sampling was performed 5 minutes after each gland excision. The surgery was completed when there was a reduction of more than 50% in baseline PTH levels (preoperative PTH measured on the day before surgery), when it fell to within the normal range (<65 pg/mL), or when the evidence of pathological gland was cleared. Before 2015, when surgery was not performed while checking IOPTH, hyperplasia of the suspected lesion was visually confirmed in the surgical field, or was confirmed with a frozen biopsy during surgery, and the operation was terminated. Otherwise, exploration was continued, and if unclear, a frozen biopsy was sent for examination to determine the presence of parathyroid gland hyperplasia, and the surgery was terminated. The final number of resected glands was confirmed by the results of a patient’s pathologic examination.
Serum Ca, iP, and PTH were measured immediately after surgery and at 3, 6, and 12 months. The normal PTH and PePTH groups were then classified, and their clinical data was compared. The normal PTH group comprised patients with normal Ca and PTH levels (serum Ca level of less than 10.5 mg/dL and PTH less than 65 pg/mL). This included those measured both immediately and 6 months postoperatively. The PePTH group was defined by patients with normal or low Ca and elevated PTH levels at least once within 6 months postoperatively, which later returned within the normal range.
Statistical analyses were performed using IBM SPSS Statistics for Windows ver. 25.0 (IBM Corp., Armonk, NY, USA). Continuous normally distributed variables were expressed as means ± standard deviations, and comparisons between the groups were performed using the Student t-test; otherwise, data were expressed as medians and interquartile ranges (Q1–Q3) and compared using the Mann-Whitney U-test. Categorical variables were summarized using counts and (percentages) and analyzed using the Pearson chi-square test. The risk analysis was performed using logistic regression analysis. A P-value of less than 0.05 was considered significant.
Go to : 
A total of 364 patients met the inclusion criteria and were included in the study. Of these, 333 patients (91.5%) were allocated to the normal PTH group and 31 (8.5%) to the PePTH group. There were no significant differences in age (P = 0.200), sex (P > 0.999), preoperative Ca levels (P = 0.215), iP levels (P = 0.295), or eGFR (P = 0.306) between the groups. However, there were significant differences in preoperative PTH levels (P < 0.001), 25OHD levels (P = 0.003), and the presence of vitamin D deficiency (P = 0.005) (Table 1).
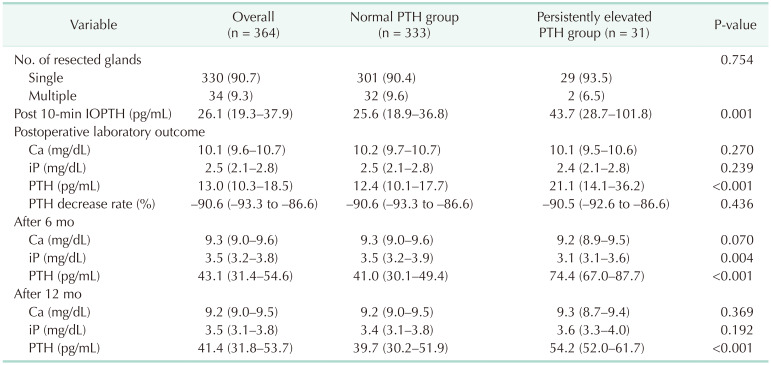
Intraoperative and postoperative pathologic and laboratory values are compared in Table 2. The number of resected glands (P = 0.754), the rate at which the PTH levels decreased immediately after surgery (P = 0.436), and the Ca levels at each stage were not significantly different. On the other hand, there were significant differences in PTH levels between the 2 groups: IOPTH (P = 0.001), postoperative PTH (P < 0.001), PTH levels after 6 months (P < 0.001), and at 12 months (P < 0.001).
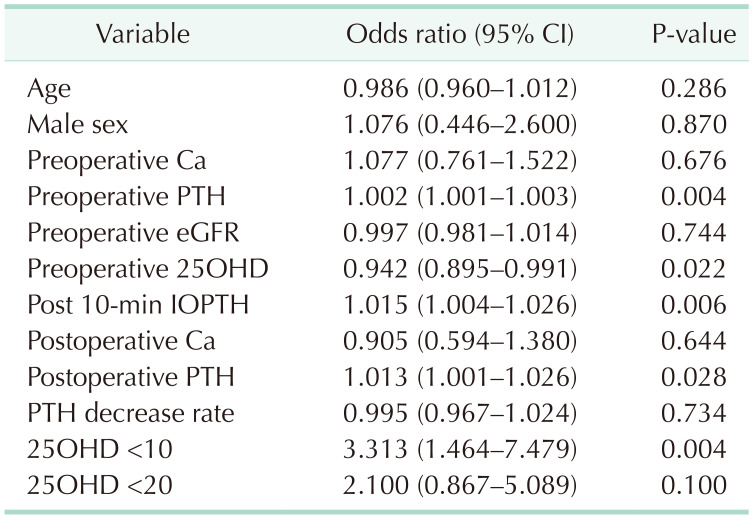
Table 3 shows the risk of PePTH according to the variables. In the group with serum 25OHD of less than 10 ng/mL, the risk of postoperative PePTH increased 3.313-folds. On the contrary, as the concentration of 25OHD increased, the odds of PePTH decreased 0.942-fold. The higher the preoperative PTH and IOPTH, the higher the risk of PePTH (1.002- and 1.015-fold, respectively).
Among the patients in the PePTH group, vitamin D deficiency was observed in 18, decreased renal function in 5, and relatively low Ca levels in 8 patients. All patients in this group had a reduction in PTH of more than 50% after surgery compared with before surgery. Eighteen patients, whose conditions were likely caused by a vitamin D deficiency, recovered to within the normal range after vitamin D injection or oral supplement at an outpatient clinic or another hospital. Of the 5 patients whose renal function was determined to be causative, 1 progressed to secondary hyperparathyroidism due to chronic kidney disease (CKD) diagnosed after surgery, and 2 were diagnosed with CKD due to kidney stone problems before surgery. The PTH levels were normalized after increasing Ca medication in patients with a sharp drop in Ca after surgery compared with before surgery.
Go to : 
PHPT surgery is associated with temporary hypocalcemia due to hypoparathyroidism, and by increasing serum PTH levels in the remaining parathyroid glands, bone demineralization is promoted to maintain serum Ca homeostasis. This phenomenon can explain the presence of PePTH even after curative surgery [10]. In patients with PHPT, constantly elevated PTH or Ca levels within 6 months after initial PTX may be suggestive of persistent or recurrent hyperparathyroidism [91011]. Here, postoperative PePTH was present in 3%–24% of the patients [8]. After successful surgery, PTH may be increased due to bone hunger, vitamin D deficiency, a sudden decrease in Ca levels, or a reduction in renal function [9111516].
Raised PTH levels after curative PTX was consistently associated with lower levels of vitamin D preoperatively [1718]. Chronic vitamin D deficiency stimulates the parathyroid gland to proliferate, leading to the formation of adenoma. Alternatively, it plays a role in promoting the growth of existing adenomas. In patients with PHPT, when serum 25OHD is lowered, various complications may occur, including parathyroid gland and tumor growth; increased serum PTH, Ca, and alkaline phosphatase levels; accelerated bone turnover; and abnormal bone formation and fractures [1920]. Hyperparathyroidism can be exacerbated by increasing vitamin D catabolism and inactivation, which is achieved by increasing 1,25-dihydroxyvitamin D levels [2122]. It is known that 1,25-dihydroxyvitamin D may affect the total vitamin D concentration, by blocking the production of vitamin D3 in the skin, inhibiting the production of 25OHD in the liver, or increasing the conversion of 25OHD to 1,25-dihydroxyvitamin D in the kidneys [23]. Rao et al. [24] reported that vitamin D deficiency may be caused by the loss of vitamin D regulation owing to the PTH gene, which is abnormally active in PHPT.
Here, the factors significantly related to elevated postoperative PTH levels were preoperative PTH levels, 25OHD levels, vitamin D deficiency, and IOPTH (Table 3). The presence of vitamin D deficiency preoperatively can lead to more severe hypocalcemia after PTX, secondary hyperparathyroidism, and the “hungry bone syndrome” [151718]. Previous studies have shown that preoperative vitamin D deficiency is also associated with postoperative hypocalcemia [2526]. In the past, vitamin D medication was restricted before surgery to avoid exacerbating hypercalcemia. However, these practices may conversely increase PTH secretion and promote bone turnover, leading to cortical bone loss. The importance of Ca supplementation has been shown in several studies. Ca supplementation within 6 months of PTX resulted in significantly fewer cases of elevated PTH levels [1627]. Tucci et al. [28] reported that patients who received sufficient Ca and vitamin D supplementation after surgery were less likely to experience postoperative PePTH.
Recurrent PHPT is defined as the reappearance of abnormal Ca and PTH levels at more than 6 months of follow-up after a period of normal levels. The causes are as follows: (1) not achieving proper localization before surgery; (2) the presence of multi-gland hyperplasia without having excised all abnormal glands; (3) genetic factors such as MEN or FIHPT; (4) ending the surgery without removing abnormal parathyroid glands; and (5) the lack of radical excision when treating parathyroid carcinoma [1429]. To address these problems, several studies have investigated the use of IOPTH for determining whether to continue surgery. At this center, IOPTH monitoring began in 2015, and this could also be a means of predicting PePTH. Here, the PePTH group showed significantly higher IOPTH levels (43.7 pg/mL) than the normal PTH group (25.6 pg/mL, P = 0.017) (Table 2).
Except for transient secondary hyperparathyroidism caused by vitamin D deficiency, low Ca levels, renal dysfunction, and recurrent PHPT were noted in 7 patients (1.9%) after follow-up for more than 6 months [41330]. The remaining patients achieved remission using only Ca supplements or vitamin D injections following their temporarily abnormal laboratory results, similar to those with temporarily elevated PTH levels. However, as mentioned in the studies described above, vitamin D deficiency can appear complexly associated with PHPT, so additional research is needed by enrolling a larger number of patients in the future [212223].
This study had some limitations. First, 56 patients were lost to follow up; hence, we do not know the effect these additional patients would have had on any recurrent rates. Second, the symptoms of patients with hyperparathyroidism were not investigated; therefore, we could not assess whether these symptoms differed pre- and postoperatively. A history of CKD, dialysis, and the use of Ca or vitamin D medication in those with low bone density was not properly considered. This may have influenced the results of our study. Third, we did not identify any indicators related to bone turnover and bone mineral density. Finally, the small number of patients with PePTH is a further limitation of this study.
Several studies have reported that the weight of the parathyroid gland may influence the postoperative outcome [38]. However, for nearly 50% of the study group, the weight of the gland was not clearly recorded in the final pathology report. Moreover, in some cases, the gland was fragmented during surgery to confirm the frozen biopsy results, and it was difficult to measure the exact weight of the gland. Therefore, these results were excluded, which could be considered another limitation of this investigation.
In conclusion, here, the presence or absence of a vitamin D deficiency before surgery was the main factor for predicting PePTH. In addition, vitamin D should be monitored in patients with temporary laboratory abnormalities within 6 months after successful surgery, and appropriate vitamin D and Ca supplementation provided as required. By doing so, additional unnecessary imaging examinations and surgeries can be avoided, thereby reducing costs and providing safer treatments for patients.
Go to : 
References
1. Rudin AV, McKenzie TJ, Wermer RA, Thompson GB, Lyden ML. Primary hyperparathyroidism: redefining cure. Am Surg. 2019; 85:214–218. PMID: 30819301.

2. Felger EA, Kandil E. Primary hyperparathyroidism. Otolaryngol Clin North Am. 2010; 43:417–432. PMID: 20510724.

3. Krawitz R, Glover A, Koneru S, Jiang J, Di Marco A, Gill AJ, et al. The significance of histologically “large normal” parathyroid glands in primary hyperparathyroidism. World J Surg. 2020; 44:1149–1155. PMID: 31773224.

4. Mallick R, Nicholson KJ, Yip L, Carty SE, McCoy KL. Factors associated with late recurrence after parathyroidectomy for primary hyperparathyroidism. Surgery. 2020; 167:160–165. PMID: 31606193.

5. Paek SH, Kim SJ, Choi JY, Lee KE. Clinical usefulness of intraoperative parathyroid hormone monitoring for primary hyperparathyroidism. Ann Surg Treat Res. 2018; 94:69–73. PMID: 29441335.

6. Grant CS, Thompson G, Farley D, van Heerden J. Primary hyperparathyroidism surgical management since the introduction of minimally invasive parathyroidectomy: Mayo Clinic experience. Arch Surg. 2005; 140:472–479. PMID: 15897443.

7. Mittendorf EA, McHenry CR. Persistent parathyroid hormone elevation following curative parathyroidectomy for primary hyperparathyroidism. Arch Otolaryngol Head Neck Surg. 2002; 128:275–279. PMID: 11886343.

8. de la Plaza Llamas R, Ramia Ángel JM, Arteaga Peralta V, García Amador C, López Marcano AJ, Medina Velasco AA, et al. Elevated parathyroid hormone levels after successful parathyroidectomy for primary hyperparathyroidism: a clinical review. Eur Arch Otorhinolaryngol. 2018; 275:659–669. PMID: 29209851.

9. Nordenström E, Westerdahl J, Bergenfelz A. Long-term follow-up of patients with elevated PTH levels following successful exploration for primary hyperparathyroidism. World J Surg. 2004; 28:570–575. PMID: 15366747.
10. Mandal AK, Udelsman R. Secondary hyperparathyroidism is an expected consequence of parathyroidectomy for primary hyperparathyroidism: a prospective study. Surgery. 1998; 124:1021–1027. PMID: 9854578.

11. Tisell LE, Jansson S, Nilsson B, Lundberg PA, Lindstedt G. Transient rise in intact parathyroid hormone concentration after surgery for primary hyperparathyroidism. Br J Surg. 1996; 83:665–669. PMID: 8689214.

12. Lou I, Balentine C, Clarkson S, Schneider DF, Sippel RS, Chen H. How long should we follow patients after apparently curative parathyroidectomy? Surgery. 2017; 161:54–61. PMID: 27863779.

13. Mazotas IG, Yen TW, Doffek K, Shaker JL, Carr AA, Evans DB, et al. Persistent/recurrent primary hyperparathyroidism: does the number of abnormal glands play a role? J Surg Res. 2020; 246:335–341. PMID: 31635835.

14. Udelsman R. Approach to the patient with persistent or recurrent primary hyperparathyroidism. J Clin Endocrinol Metab. 2011; 96:2950–2958. PMID: 21976743.

15. Wang TS, Ostrower ST, Heller KS. Persistently elevated parathyroid hormone levels after parathyroid surgery. Surgery. 2005; 138:1130–1136. PMID: 16360400.

16. Carty SE, Roberts MM, Virji MA, Haywood L, Yim JH. Elevated serum parathormone level after “concise parathyroidectomy” for primary sporadic hyperparathyroidism. Surgery. 2002; 132:1086–1093. PMID: 12490859.

17. Bergenfelz A, Valdemarsson S, Tibblin S. Persistent elevated serum levels of intact parathyroid hormone after operation for sporadic parathyroid adenoma: evidence of detrimental effects of severe parathyroid disease. Surgery. 1996; 119:624–633. PMID: 8650602.

18. Denizot A, Pucini M, Chagnaud C, Botti G, Henry JF. Normocalcemia with elevated parathyroid hormone levels after surgical treatment of primary hyperparathyroidism. Am J Surg. 2001; 182:15–19. PMID: 11532408.

19. Silverberg SJ, Shane E, Dempster DW, Bilezikian JP. The effects of vitamin D insufficiency in patients with primary hyperparathyroidism. Am J Med. 1999; 107:561–567. PMID: 10625024.

20. Rao DS, Agarwal G, Talpos GB, Phillips ER, Bandeira F, Mishra SK, et al. Role of vitamin D and calcium nutrition in disease expression and parathyroid tumor growth in primary hyperparathyroidism: a global perspective. J Bone Miner Res. 2002; 17 Suppl 2:N75–N80. PMID: 12412781.
21. Clements MR, Davies M, Fraser DR, Lumb GA, Mawer EB, Adams PH. Metabolic inactivation of vitamin D is enhanced in primary hyperparathyroidism. Clin Sci (Lond). 1987; 73:659–664. PMID: 3690980.

22. Clements MR, Davies M, Hayes ME, Hickey CD, Lumb GA, Mawer EB, et al. The role of 1,25-dihydroxyvitamin D in the mechanism of acquired vitamin D deficiency. Clin Endocrinol (Oxf). 1992; 37:17–27. PMID: 1424188.

23. Silverberg SJ. Vitamin D deficiency and primary hyperparathyroidism. J Bone Miner Res. 2007; 22 Suppl 2:V100–V104. PMID: 18290710.

24. Rao D, Han ZH, Phillips E, Palnitkar S, Parfitt A. Loss of calcitriol receptor expression in parathyroid adenomas: implications for pathogenesis (Abstract). J Bone Miner Res. 1997; 12:S107.
25. Boudou P, Ibrahim F, Cormier C, Sarfati E, Souberbielle JC. A very high incidence of low 25 hydroxy-vitamin D serum concentration in a French population of patients with primary hyperparathyroidism. J Endocrinol Invest. 2006; 29:511–515. PMID: 16840828.

26. Stewart ZA, Blackford A, Somervell H, Friedman K, Garrett-Mayer E, Dackiw AP, et al. 25-hydroxyvitamin D deficiency is a risk factor for symptoms of postoperative hypocalcemia and secondary hyperparathyroidism after minimally invasive parathyroidectomy. Surgery. 2005; 138:1018–1026. PMID: 16360386.

27. Dhillon KS, Cohan P, Darwin C, Van Herle A, Chopra IJ. Elevated serum parathyroid hormone concentration in eucalcemic patients after parathyroidectomy for primary hyperparathyroidism and its relationship to vitamin D profile. Metabolism. 2004; 53:1101–1106. PMID: 15334367.

28. Tucci JR. Vitamin D therapy in patients with primary hyperparathyroidism and hypovitaminosis D. Eur J Endocrinol. 2009; 161:189–193. PMID: 19383807.

29. Chen H, Wang TS, Yen TW, Doffek K, Krzywda E, Schaefer S, et al. Operative failures after parathyroidectomy for hyperparathyroidism: the influence of surgical volume. Ann Surg. 2010; 252:691–695. PMID: 20881776.
30. Alhefdhi A, Schneider DF, Sippel R, Chen H. Recurrent and persistence primary hyperparathyroidism occurs more frequently in patients with double adenomas. J Surg Res. 2014; 190:198–202. PMID: 24656398.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download