This article has been
cited by other articles in ScienceCentral.
Abstract
Purpose
Analyses on pure laparoscopy in donor hepatectomies, including the middle hepatic vein (MHV), are still scarce. This study aimed to compare the outcomes of donor right hepatectomy, including the MHV, when performed laparoscopically with conventional open surgery.
Methods
Data from living donors who underwent donor right hepatectomy between January 2012 and December 2020 were retrospectively analyzed. The intraoperative and postoperative complication rates of the pure laparoscopic donor right hepatectomy (PLDRH) with MHV inclusion (PLDRHM) group were compared with the conventional open donor right hepatectomy with MHV inclusion (CDRHM) group and the PLDRH without MHV inclusion [PLDRHM(–)] group.
Results
Compared to the CDRHM group, the PLDRHM group had a longer bench time (P < 0.001) and higher Δ%, calculated as [(preoperative value – postoperative value)/preoperative value] × 100, of AST (P < 0.001), ALT (P < 0.001), and total bilirubin (P = 0.023), but shorter hospital stay (P = 0.004) and a lower rate of complications (P = 0.005). Compared to the PLDRHM(–) group, the PLDRHM group had fewer male donors (P < 0.001) and a lower body mass index (P < 0.001), estimated total liver volume (P < 0.001), and real graft weight (P < 0.001). Results of laboratory changes, hospital stays, and complication rates were similar between the 2 groups.
Conclusion
PLDRH with the inclusion of the MHV in selected donors and recipients is feasible and safe when performed by surgeons experienced in laparoscopic surgery, with favorable complication rates compared to CDRHM and PLDRHM(–).
Keywords: Hepatectomy, Laparoscopy, Liver transplantation
INTRODUCTION
Laparoscopic hepatectomy reportedly involves fewer complications, shorter hospital stays, and less intraoperative blood loss than conventional open liver surgery [
1]. The benefits of laparoscopic surgery have been well demonstrated especially among transplantation donors, where the donor is usually a healthy individual without previous scars. It has been reported that donor satisfaction in terms of operative wounds is superior compared to the conventional open method [
2]. Similar benefits of less blood loss, fewer complications, and shorter hospital stays have also been reported with laparoscopic donor hepatectomy in liver transplantation [
3].
Since donor hepatectomy involves possible complications such as bleeding, biliary stricture, and bile leak, the most critical concern in the transplantation process is minimizing the risk of complications [
4]. Laparoscopic surgery must be performed without compromising donor safety, making laparoscopic donor hepatectomy a technically challenging surgery.
The first case of laparoscopic donor hepatectomy was reported in 2002 for a living donor and pediatric recipient [
5]. With the emergence of minimally invasive surgery, significant advancements have been made in laparoscopic techniques, and varying extents of hepatectomies are now being safely practiced [
3]. Pure laparoscopic donor right hepatectomy (PLDRH) was first performed in 2010 [
6] and has been increasingly adopted for living donor liver transplantation, with preliminary studies demonstrating excellent safety and favorable outcomes at experienced centers [
678]. Advances in technology such as the development of the 3-dimensional (3D) laparoscope, flexible scope, and indocyanine green near-infrared fluorescence ushered in a new era of pure laparoscopic donor hepatectomy (PLDH). Since 2014, several studies and analyses have proved the safety and feasibility of PLDH [
91011].
The first case of procuring the middle hepatic vein (MHV) in donor hepatectomy was described by Lo et al. in 1997 [
12]. MHV inclusion in the liver graft benefits the recipient since venous congestion of segments V and VIII in a graft lacking drainage to the MHV may lead to graft dysfunction or failure. However, the inclusion of the MHV in the liver graft has been highly debated, mainly due to technical difficulties and donor safety issues [
13]. Procuring the MHV may also cause problems in venous drainage of the anterior section of the remnant liver of the donor, resulting in delayed regeneration [
14]. This problem was minimized by maintaining a remnant liver volume above 30% [
15]. Thus, the decision regarding the inclusion of MHV should be made after considering the graft and remnant liver volumes, along with the metabolic demand of the recipient. Several reports have suggested that MHV inclusion can be safely performed in most donors [
16].
To date, only a few studies have investigated the safety of donor hepatectomy including MHV, performed laparoscopically. In this study, we report our findings from PLDRH with MHV inclusion (PLDRHM) and compare the perioperative complication rates with the conventional open method (CDRHM). We also compared the differences in outcomes between PLDRHM and PLDRH without MHV inclusion [PLDRHM(–)].
METHODS
The Institutional Review Board of Seoul National University Hospital approved this study (No. H-2106-041-1226). The requirement for informed consent was waived because of the retrospective nature of the study. The study was performed according to the Helsinki Declaration.
All donor data were collected from electronic medical records and analyzed retrospectively. The study population was divided into the PLDRHM, PLDRHM(–), and CDRHM groups. Since most donor hepatectomies were performed laparoscopically after the first case in November 2015, we selected each group from a different period to reduce selection bias. The CDRHM group was selected from January 2012 to October 2015, and the PLDRHM and PLDRHM(–) groups were selected from November 2015 to December 2020. All patients were informed of the novelty of the procedure, the risks of complications, especially when anatomical variations were present, and that further studies are needed to investigate the benefits of the procedure. There were no selection criteria for PLDH after March 2016. However, there some conventional open hepatectomies were performed after March 2016. In most of these open cases, patients declined the new procedure after the explanation. In some cases, the indocyanine green camera or 3D flexible scope was unavailable on the day of surgery. A total of 52 patients who received non-right (left, left lateral, and right posterior section) liver grafts and patients treated using 39 laparoscopy-assisted procedures were excluded.
Our center has published multiple articles on PLDH, including both right and left grafts [
8917]. Specifics and surgical techniques, such as port placement and indocyanine green near-infrared fluoroscopy, are described in our previous articles in detail [
18].
We analyzed age, sex, body mass index (BMI), graft weight, remnant liver (%), operative time, warm ischemic time, bench work time, estimated blood loss, the presence of anatomical variations (more than 1 bile duct, portal vein, or hepatic artery), postoperative serum lab-level change (ΔAST%, ΔALT%, Δtotal bilirubin%, Δhemoglobin [Hb]%), and duration of admission. The outcomes of the PLDRHM group were compared individually with those of both the CDRHM group (analysis 1) and the PLDRHM(–) group (analysis 2).
Statistical analysis
Results are reported as mean values with standard deviations, and in the CDRHM group, results are shown as medians with ranges. Continuous variables were compared using Student t-test or the Mann-Whitney U-test. Categorical variables were compared using the chi-square test and Fischer exact test. Statistical significance was set at P < 0.05. All statistical analyses were performed using IBM SPSS Statistics ver. 25 (IBM Corp., Armonk, N.Y., USA).
RESULTS
Fig. 1 shows the study design. Of the 914 liver donors who underwent surgery between January 2012 and December 2020, 39 laparoscopy-assisted cases, 93 cases of open donor hepatectomy after 2015, 52 non-right liver graft donors, and 289 donors who provided right liver grafts without MHV inclusion who underwent conventional open surgery were excluded from the study.
Fig. 1
Study design. MHV, middle hepatic vein; CDRHM, conventional open donor right hepatectomy with MHV inclusion; PLDRHM, pure laparoscopic donor right hepatectomy with MHV inclusion; PLDRHM(–), pure laparoscopic donor right hepatectomy without MHV.


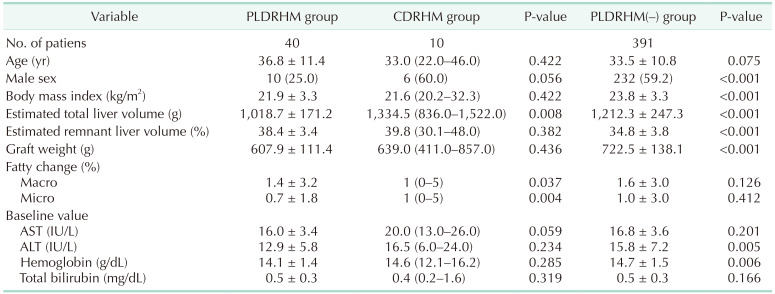
The baseline demographics and primary outcomes of the 40 patients who underwent PLDRHM are summarized in
Table 1. The mean age was 36.8 years, 25.0% were male, and patients had a mean BMI of 21.9 kg/m
2. The mean total liver volume was 1,018.7 g, with a mean graft weight of 607.9 g and a graft-to-recipient weight ratio (GRWR) of 0.9.
Table 1
Baseline demographics of the PLDRHM, CDRHM, and PLDRHM(–) groups

Recipient characteristics are summarized in
Supplementary Table 1. The mean age was 54.3 years, 80.0% were male, and the mean BMI was 25.6 kg/m
2. The mean Model for End-Stage Liver Disease (MELD) score for recipients was 11.7, and the GRWR was 0.9. Exactly 65% of the patients had hepatocellular carcinoma. We divided the recipients’ major (Clavien-Dindo grade III or above) complications into early (within 1 month of surgery) or late complications. Early major complications occurred in 22.5% of patients, and late major complications occurred in 45.0% of patients. The mean hospital stay was 20.6 days.
Analysis 1: PLDRHM vs. CDRHM
Analysis 1 revealed that the PLDRHM group was predominantly female (25.0% of male), while the CDRHM group was predominantly male (60.0%). The PLDRHM group had a smaller total liver volume (1,018.7 g
vs. 1,334.5 g, P < 0.001); however, the estimated remnant liver volume and graft weight were similar between the 2 groups. The PLDRHM group had a longer bench time of 56.3 minutes compared to 20.0 minutes in CDRHM (P < 0.001) (
Table 2). There was no significant difference in operative time between the 2 groups. Although not statistically significant, the estimated blood loss in PLDRHM tended to be significantly less compared to CDRHM (202.5 mL
vs. 235.0 mL, P = 0.067). The PLDRHM group showed a more significant percentage increase in serum AST (P < 0.001), ALT (P < 0.001), and total bilirubin (P = 0.023) compared to the CDRHM group, which was calculated as [(preoperative value – postoperative value)/preoperative value] × 100. The mean change in hemoglobin level was not statistically significant. The duration of hospital stay was shorter in the PLDRHM group than in the CDRHM group (7.0 days
vs. 8.0 days, P = 0.004).
Table 2
Postoperative outcomes of the PLDRHM, CDRHM, and PLDRHM(–) groups
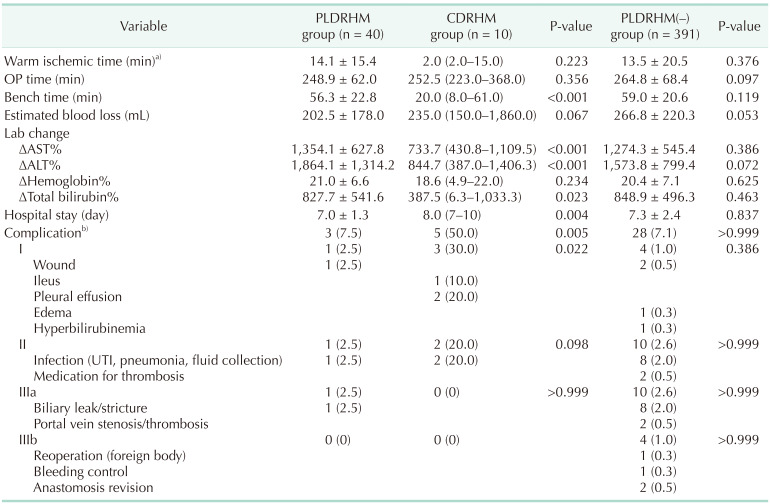

The PLDRHM group experienced significantly fewer total complications (3 of 40, 7.5%) than the CDRHM group (5 of 10, 50.0%) with 1 case of a Clavien-Dindo grade I, 1 case of a grade II, and 1 case of a grade III complication. These cases were of wound problems, infection due to intraabdominal fluid collection resolved by intravenous (IV) antibiotic therapy, and biliary stricture requiring endoscopic biliary stenting, respectively. Of all the CDRHM group complications, 3 of 5 were grade I, and 2 of 5 were grade II. There was 1 case of postoperative ileus, 1 case of pneumonia resolved by conservative care and oral antibiotic therapy, 2 cases of pleural effusion resolved by conservative care, and intraabdominal fluid collection requiring IV antibiotic therapy. Sub-analysis categorized by Clavien-Dindo grade showed only a significant difference in grade I complications (P = 0.022).
Analysis 2: PLDRHM vs. PLDRHM(–)
In analysis 2, the PLDRHM(–) group was also mostly male (59.2%) with a higher mean BMI of 23.8 kg/m
2 (P < 0.001). The GRWR was higher in the PLDRHM(–) group, with a mean value of 1.2 compared to 0.9 in the PLDRHM group (P < 0.001). Similar to analysis 1, the PLDRHM group had a smaller estimated total liver volume (1,018.7 g
vs. 1,212.3 g, P < 0.001), larger estimated remnant liver volume (38.4%
vs. 34.8%, P < 0.001), and lighter real graft (607.9 g
vs. 722.5 g, P < 0.001) than the PLDRHM(–) group. Operative time, warm ischemic time, bench time, intraoperative estimated blood loss, and change in postoperative laboratory results did not statistically significantly differ between the 2 groups (
Table 2). There were 28 complications (7.2%) in the PLDRHM(–) group, including 4 cases of grade I (1.0%), 10 cases of grade II (2.6%), 10 cases of grade IIIa (2.6%), and 4 cases of grade IIIb complications (1.0%). These included 2 cases of wound complications, 1 case of perineal edema, 1 case of hyperbilirubinemia, 2 cases of biloma treated medically, 1 case of intraabdominal hematoma, 1 case of urinary tract infection, 3 cases of intraabdominal fluid collection requiring antibiotics, 1 case of portal vein thrombus treated with aspirin, 1 case of pulmonary thromboembolism, 8 cases of biliary leak or stricture requiring endobiliary stenting or percutaneous catheter drainage (PCD) insertion, 2 cases of portal vein thrombosis, 1 case of surgical foreign body removal (pain buster), 1 case of bleeding control, and 2 cases of hepatic vein anastomosis revision. There were no statistically significant differences in terms of complications between the 2 groups.
DISCUSSION
Data and analyses of laparoscopic donor hepatectomy have been extensively explored earlier, and there is much evidence supporting its safety and feasibility [
11]. However, the safety of PLDRH including MHV has not yet been analyzed separately.
Analysis 1 (CDRHM
vs. PLDRHM) showed that the CDRHM group had larger total and remnant liver volumes than the PLDRHM group; this might lead one to presume that larger livers are more challenging to operate laparoscopically. However, at our institution, since its first implementation in November 2015, we have performed PLDRH in more than 90% of all the cases without any specific selection criteria from March 2016. This indicates that liver volume is not a determinant of the feasibility of PLDRH. Similar results have been reported in our previous study regarding graft volume [
10]. The larger liver volumes in the CDRHM group can be explained by the relatively higher female ratio (30 of 40 in PLDRHM
vs. 4 of 10 in CDRHM) in the PLDRHM group. It is possible that the propagation of PLDH would lead to an increase in female donors.
The CDRHM group had a significantly shorter bench time than the PLDRHM group. This finding is consistent with our previous study, which, although limited to right donor hepatectomies without MHV inclusion, reported longer bench times in PLDH hepatectomies than in conventional open donor hepatectomies [
19]. We believe that this finding can be attributed to shorter vasculature and bile duct length in the laparoscopically harvested liver grafts as a result of instruments such as clips and staplers, thus requiring longer bench times to improve the graft quality. Our results show that the MHV-included cases show a similar trend.
Consistent with our previous studies that focused only on right hepatectomies without MHV inclusion, the PLDRHM group showed a greater Δ% value for AST, ALT, and total bilirubin than the CDRHM group, and differences in hemoglobin levels were not statistically significant. We thought that technical difficulties in procuring the MHV could lead to a relatively higher blood loss or Δ%Hb value; however, we did not observe such in our group. In contrast, the magnified laparoscopic view could have helped in procurement. In summary, the bleeding risk did not seem to be worse in the PLDRHM group. Hospital stay was also shorter by 0.97 days in the PLDRHM group, similar to our previous analyses on laparoscopy versus open hepatectomies [
89].
The 2 groups showed a significant difference in Clavien-Dindo grade I complications, with 3 of 10 patients in the CDRHM group (intraabdominal fluid collection cases treated with IV antibiotics, postoperative ileus, and conservatively treated pneumonia) and 1 of 40 in the PLDRHM group (intraabdominal fluid collection cases treated with IV antibiotics) exhibiting complications. There were no statistically significant differences in terms of the major complications, and there were no grade IV–V complications. Interestingly, our previous data on PLDRH showed a shorter operative time than the open group; however, this was not the case in our collective data on donor right hepatectomy with MHV inclusion. The average operative time was shorter in the PLDRHM group than that in the CDRHM group (248.9 minutes vs. 252.5 minutes); however, the small sample size may have affected the analysis.
As expected, the PLDRHM(–) group had a higher mean BMI, larger total liver volume, and higher graft weight than the PLDRHM group. This finding aligns well with our hypothesis because inclusion of the MHV with the liver graft is indicated when there is sufficient remnant liver volume and the GRWR is relatively small. As a result, the odds of the donor having a smaller BMI and a smaller liver volume are increased. There was no significant difference in operative or bench times, and lab changes were similar between the PLDRHM and PLDRHM(–) groups. The need for polytetrafluoroethylene graft venous reconstruction to save the V5 and V8 branches of the MHV in PLDRHM(–) led us to wonder whether the bench time would be relatively long; however, this was not observed in our study. This could be because PLDRHM still requires venous reconstruction to connect the MHV and right hepatic vein into 1 common draining vein for convenient venous anastomosis. In addition, the operative time was not affected by the inclusion or exclusion of MHV in the graft, indicating that the technical aspect was not a significant rate-limiting factor. Contrary to our concern, Δ% values of AST, ALT, and hemoglobin did not statistically significantly differ between the groups. Donor complications also did not show statistically significant differences overall or in any Clavien-Dindo grade.
Although not statistically significant, 2.5% of the PLDRHM group and 2.6% of the PLDRHM(–) group had complications above Clavien-Dindo grade IIIa. The majority were biliary leaks (intraabdominal bilomas) requiring PCD insertion or biliary strictures and either external drainage with percutaneous transhepatic biliary drainage, or an endoscopically placed internal biliary stent/drain. The much larger PLDRHM(–) group also had 4 cases (1.0%) of grade IIIb complications: 1 case of postoperative bleeding control, 2 cases of anastomosis revision, and 1 peculiar case of a surgical tool (pain buster) left in the abdomen requiring surgical foreign body removal. The open conventional group had no complications above grade IIIa, but we suspect that this is owing to the small sample.
Our study had several limitations. The study design and analysis were performed retrospectively, making some variables prone to bias. The CDRHM group was small (n = 10), probably due to the onset of laparoscopy and MHV-included hepatectomies being relatively close in time. Additionally, this study used data from a single center, which means that our results may not be generalizable and interpretation may be difficult to a larger and diverse population. Although liver function test changes were included as variables, specific parameters related to MHV inclusion, especially those involved in liver congestion, were not included in the analysis. Heterogeneity in demographics and volumetric parameters may have resulted in bias and, thus, the results should be interpreted carefully. Despite these limitations, to the best of our knowledge, our study is the first to compare laparoscopy and conventional open donor hepatectomy including MHV. Randomized controlled studies may be difficult to perform for this comparison, but further studies with larger samples and propensity score matching would be beneficial.
In conclusion, with laparoscopic donor hepatectomy gradually becoming the standard procedure, donor right hepatectomies including the MHV can be safely performed using pure laparoscopy in selected donors and recipients, with outcomes comparable to the conventional open method or right hepatectomies without MHV inclusion. Long-term multicenter outcomes, including complications or variables more specific to MHV inclusion, would provide stronger evidence to aid decision-making.






 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download