Abstract
Background
High-density lipoprotein cholesterol (HDL-C) plays an important role in the reverse cholesterol transport pathway and prevents atherosclerosis-mediated disease. It has also been suggested that HDL-C may be a protective factor against cancer. However, an inverse correlation between HDL-C and cancer has not been established, and few studies have explored thyroid cancer.
Methods
The study participants received health checkups provided by the Korean National Health Insurance Service from 2009 to 2013 and were followed until 2019. Considering the variability of serum HDL-C level, low HDL-C level was analyzed by grouping based on four consecutive health checkups. The data analysis was performed using univariate and multivariate Cox proportional hazard regression models.
Results
A total of 3,134,278 total study participants, thyroid cancer occurred in 16,129. In the crude model, the hazard ratios for the association between repeatedly measured low HDL-C levels and thyroid cancer were 1.243, 1.404, 1.486, and 1.680 (P for trend <0.01), respectively, which were significant even after adjusting for age, sex, lifestyle factors, and metabolic diseases. The subgroup analysis revealed that low HDL-C levels likely had a greater impact on the group of patients with central obesity (P for interaction= 0.062), high blood pressure (P for interaction=0.057), impaired fasting glucose (P for interaction=0.051), and hyperlipidemia (P for interaction=0.126).
High-density lipoprotein cholesterol (HDL-C) plays an important role in the reverse cholesterol transport pathway by complementing low-density lipoprotein cholesterol (LDL-C), which contributes to the systemic circulation of cholesterol [1]. HDL-C protects against cardiovascular disease [2,3]. Furthermore, it has also been suggested that HDL-C may be a protective factor against cancer [4,5]. According to a previous study on the effects of HDL-C on cancer development, an inverse association between HDL-C and cancer was found to be particularly strong in hematologic malignancies [6]. An association between low HDL-C levels and the risk of cancer has also been reported in solid cancers, such as breast [7] and lung cancer [8]. However, an inverse correlation between HDL-C and cancer has not been established in all types of cancers, and there have been few studies on thyroid cancer and HDL-C levels.
The incidence of thyroid cancer is generally increasing, and an increase due to the development and greater availability of examination technology is suggested as the main cause [9,10]. However, researchers must also pay attention to lifestyle and environmental factors because the prevalence of large thyroid cancer is also increasing [11]. Many researchers have found a pattern of development in thyroid cancer similar to that of metabolic disease. Previous studies have reported an association between obesity and thyroid cancer [12-15]. Moreover, the risk of thyroid cancer decreased with weight loss [16]. A subsequent study found that metabolic syndrome may also be a risk factor for thyroid cancer [17]. Since HDL-C is one of the components of metabolic syndrome, we hypothesized that there is a relationship between low HDL-C levels and the occurrence of thyroid cancer and therefore considered thyroid cancer to be a disease affected by metabolic status.
The purpose of this study was to confirm the association between low HDL-C levels and the occurrence of thyroid cancer in a nationwide population-based cohort. In particular, we used repeated health examination data to evaluate the cumulative effect of HDL-C.
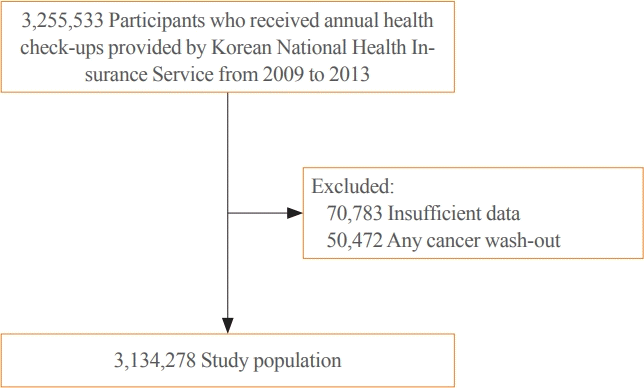
The analysis was conducted using health checkup data from the database of the Korean National Health Insurance Service, which manages medical expenses for 97.2% of the Korean population [18]. Informed consent was waived considering the retrospective design of the study and the characteristics of anonymous data provided through public institutions, and the study protocol was monitored by the Institutional Review Board of Yeouido St. Mary’s Hospital (SC21ZESI0041). The study participants received health checkups from 2009 to 2013 and were followed until 2019. Participants who underwent four consecutive health checkups were eligible. Cases with a diagnosis of any cancer at the baseline were excluded in the screening from 2012 to 2013.
Standardized questionnaires were filled out by the participants and used to assess age, sex, medical history, and social history, including alcohol consumption, cigarette smoking, regular exercise, and income status. Alcohol consumption was grouped based on an average daily alcohol intake of 30 g. Smoking status was defined as never, ex, and current smoking; those who smoked more than 50 cigarettes in their lifetime but who had quit were classified as ex-smokers and those who were still smoking as current smokers. Regular exercise was defined as daily light exercise 5 days a week or vigorous exercise 3 days a week. A low income status was defined as those who were below the 20% income percentile or eligible for medical aid.
Height and weight were measured using an electronic scale. Waist circumference was measured at an intermediate position below the lowest rib and above the pelvic bone by an experienced examiner, and systolic blood pressure (SBP) and diastolic blood pressure (DBP) were obtained after 5 minutes of rest. After fasting for more than 8 hours, a blood sample was taken, and fasting glucose, total cholesterol, triglyceride, LDL-C, and HDL-C levels were measured. Chronic disease was defined as SBP ≥140 mm Hg or DBP ≥90 mm Hg or patients currently prescribed medication for high blood pressure, fasting glucose ≥126 mg/dL or patients currently prescribed medication for diabetes, and total cholesterol ≥240 mg/dL or patients currently prescribed lipid-lowering drugs.
Groups were defined as the number of times the HDL-C level was measured as <40 mg/dL for men and <50 mg/dL for women in four consecutive health checkups. The outcome measure of all analyses was newly developed thyroid cancer, which was defined by cancer registration codes that are classified as severe disease for insurance reimbursement.
Continuous variables are described as means and standard deviations, and categorical variables are presented as numbers and percentages. Comparisons between groups were analyzed by t tests for continuous variables and chi-square tests for categorical variables. The cancer incidence was expressed in person-years (per 1,000). A survival analysis was performed by dividing groups based on the number of times a low HDL-C level was confirmed considering the variability in repeated HDL-C levels and the cumulative effect. This analysis was performed using univariate and multivariate Cox proportional hazard regression models, and the results were expressed as adjusted hazard ratios (HRs) and their 95% confidence intervals (CIs). The statistical analysis was performed with SAS version 9.3 (SAS Institute Inc., Cary, NC, USA) and R version 3.2.3 (the R Foundation for Statistical Computing, Vienna, Austria), where two-sided P values <0.05 (<0.20 for interaction analysis) were considered statistically significant.
A total of 3,255,533 people underwent annual health checkups provided by the Korean National Health Insurance Service from 2009 to 2013. We excluded 70,783 patients with insufficient information and 50,472 patients with a confirmed any cancer code in 2012 and 2013 (Fig. 1). In a follow-up assessment conducted until December 2019, thyroid cancer was diagnosed in a total of 16,129 patients. The mean age of all patients was 43.6 years. The participants were divided into groups by the number of times a low HDL-C level was confirmed in four consecutive health checkups (Fig. 2). The more frequently the low HDL-C level was measured, the older the patient was, the higher the prevalence of metabolic diseases, and the higher the proportion of low-income status (Table 1).
In the crude Model 1, the HR for the occurrence of thyroid cancer were 1.243 (95% CI, 1.191 to 1.129), 1.404 (95% CI, 1.336 to 1.476), 1.486 (95% CI, 1.407 to 1.569), and 1.680 (95% CI, 1.596 to 1.769), respectively (P for trend <0.001). We adjusted for age and sex in Model 2, for age, sex, smoking, drinking, regular exercise, and income status in Model 3, and for age, sex, smoking, drinking, regular exercise, income status, waist circumference, diabetes, high blood pressure, and hyperlipidemia in Model 4, sequentially. The HR of thyroid cancer occurrence in Model 4, which corrected all relevant factors, was 1.130 (95% CI, 1.082 to 1.179), 1.231 (95% CI, 1.170 to 1.295), 1.260 (95% CI, 1.192 to 1.333), and 1.413 (95% CI, 1.337 to 1.494), respectively (P for trend <0.001) (Table 2, Fig. 3).
A subgroup analysis was additionally performed on the factors of metabolic syndrome with the model adjusted for age, sex, drinking, smoking, regular exercise, and income. Low HDL-C levels were thought to have a greater impact on the group of patients with central obesity (P for interaction=0.062), high blood pressure (P for interaction=0.057), impaired fasting glucose (P for interaction=0.051), and hyperlipidemia (P for interaction=0.126). The HR for the occurrence of thyroid cancer was 1.714 (95% CI, 1.529 to 1.921) when diabetic patients demonstrated low HDL-C levels in four consecutive screenings, and it was for hyperlipidemia patients (Table 3).
This study analyzed the association between low HDL-C levels and the occurrence of thyroid cancer through health checkup data from the Korean National Health Insurance Service. Considering the variability of serum HDL-C level, low HDL-C level was analyzed by grouping based on four consecutive health checkups. A repeatedly low level of HDL-C increased the incident risk of thyroid cancer. In the crude model, the HRs for the occurrence of thyroid cancer were 1.243 (95% CI, 1.191 to 1.129), 1.404 (95% CI, 1.336 to 1.476), 1.486 (95% CI, 1.407 to 1.569), and 1.680 (95% CI, 1.596 to 1.769) according to the number of repeated low HDL-C levels, respectively (P for trend <0.001). The association between low HDL-C levels and the risk of thyroid cancer was significant after adjusting for age, sex, lifestyle factors, and metabolic diseases, which are other variables that may affect the development of thyroid cancer (Table 2, Fig. 3).
Previous studies provided controversial results regarding the association between HDL-C and thyroid cancer. A European study reported that the association of cancer with HDL-C level was not significant in endocrine-related cancers, including thyroid cancer [6]. However, they did not separately classify thyroid cancer in their analysis of endocrine-related cancers. Therefore, various types of genetically occurring carcinomas in endocrine-related cancers may have been included. We also did not subgroup the histologic type of thyroid cancer in this study; it was assumed HDL-C mainly influenced the development of the differentiated thyroid cancer type considering the prevalence of thyroid cancer in Korea, as 95% are differentiated thyroid cancers [19]. This fact is worth noting along with the fact that the worldwide incidence of papillary-type thyroid cancer is steadily increasing [20].
Another study proposed that an increase in thyroid nodules was associated with insulin resistance, which was independent of any changes in HDL-C [21]. However, that finding differs from this study in that the outcome of the study was not thyroid cancer, given that most thyroid nodules are benign [22]. In fact, a significantly lower HDL-C level was confirmed in the thyroid cancer patient group in a single-center study conducted in China [23]. To evaluate insulin resistance, we carried out additional analysis with body mass index (BMI). BMI was adjusted for instead of waist circumference, and the association between low HDL-C and the risk of thyroid cancer was confirmed (Supplemental Table S1). In addition, no significant associations were observed when interaction analysis was performed on the group divided using a cut-off of BMI 25 kg/m2 (Supplemental Table S2). Therefore, the association between HDL-C and thyroid cancer was significant even when considering insulin resistance in this study population.
In the subgroup analysis, low HDL-C levels were thought to play a more important role in the group of patients with metabolic disease (Table 3). Previous studies have reported that low HDL-C levels increase the cancer risk in diabetic patients [24]. Another study found that the risk of thyroid cancer is further increased in a metabolically unhealthy obese population [25]. In the results of this study, the risk of thyroid cancer in the hyperlipidemia group with four consecutive low HDL-C levels was almost twice that of the reference population (Table 3). Since metabolically unhealthy patients with high blood pressure, diabetes, and hyperlipidemia undergo repeated hospital visits and blood tests, it is thought that their cancer risk can be predicted by monitoring HDL-C levels. Intensive lipid-lowering therapy is recommended for the prevention of cardiovascular disease in a number of patients with metabolic diseases, and a previous study reported the effect of HDL-C level on the cancer incidence while using lipid-lowering drugs [26]. The use of statin-based drugs increases HDL-C levels, and this effect was greater in patients with diabetes and hyperlipidemia in a previous study [27]. Therefore, it is possible that the use of statins in a metabolically unhealthy population is a mechanism that helps prevent cancer.
The biologic mechanisms underlying the link between lipid metabolism and cancer are not well understood. In the presence of insulin resistance associated with obesity, the lipid profile often shows a change with an increase in triglycerides and a decrease in HDL-C [28]. Therefore, insulin resistance may be one factor that can explain the association between low HDL-C and thyroid cancer. An increased risk of thyroid cancer has been reported in diabetic patients [29]; insulin resistance not only causes changes in lipid profiles but may also be a factor that increases the risk of thyroid cancer. In addition, changes in thyroid function tend to decrease thyroid hormone levels and increase thyroid stimulating hormone (TSH) to control energy expenditure in a sustained positive energy status since thyroid hormones are involved in the thermogenesis of the entire body [30,31]. Metabolic status is also associated with autoimmune thyroid disease [32], which may be the cause of an increase in TSH [33]. TSH affects the growth and differentiation of thyroid cells [34,35]; higher levels of TSH are seen in patients with thyroid cancer compared to patients with benign thyroid disease [36]. An elevated TSH level is associated with hyperlipidemia [37,38], and TSH may be one of the etiologies that explains the low HDL-C often documented in thyroid cancer.
From a molecular point of view, various hypotheses have been suggested to explain the protective effect of HDL-C on cancer. First, HDL-C contains an antioxidant enzyme called paraoxonase-1, which is thought to inhibit tumorigenesis by reducing oxidative stress in peripheral tissues [39]. Second, HDL-C is thought to affect the expression level of adenosine triphosphate-binding cassette (ABC) transporters in the cell membrane. The overexpression of ABC transporters has been confirmed in a number of carcinomas, which is thought to be related to more aggressive features and increased treatment resistance [40]. Third, the HDL-C receptor, a scavenger receptor type B1, is thought to reflect the characteristics of rapidly proliferating cancer cells due to its increased expression on the surface of tumor cells. It is also suggested as a mechanism for the circulating HDL-C level to be identified as low [41]. The molecular mechanisms of the role of HDL-C in cancer require ongoing research and may someday be used to develop novel diagnostic biomarkers and therapeutic targets.
This study has a limitation in that it was only conducted in the Korean population. Korea is composed of a single ethnic group residing in an iodine-replete area [42], so it was not possible to determine differences according to demographics. The association of thyroid disease with lipid profile or thyroid cancer could not be considered because thyroid function test results or use of thyroid-related drugs, such as levothyroxine and anti-thyroid drugs, could not be confirmed in the health examination dataset. In addition, the possibility that low HDL-C is caused by cancer cannot be excluded from the results of this study. Many thyroid cancer patients live with cancer for years before receiving a clear diagnosis, as small thyroid nodules are not indicated for biopsy. However, this study has the strength of a low possibility of specific bias because we used data from a large-scale population managed by national institutions.
In conclusion, repeatedly measured low HDL-C can be considered a risk factor related to cancer as well as vascular disease. Low HDL-C levels were associated with thyroid cancer risk, and this correlation was stronger in the metabolically unhealthy population.
Supplementary Information
Supplemental Table S1.
Low High-Density Lipoprotein Cholesterol Levels and the Risk of Thyroid Cancer
Supplemental Table S2.
Effects of Low High-Density Lipoprotein Cholesterol Levels on Thyroid Cancer Incidence According to Obesity
REFERENCES
2. Assmann G, Gotto AM Jr. HDL cholesterol and protective factors in atherosclerosis. Circulation. 2004; 109(23 Suppl 1):III8–14.

3. McTaggart F, Jones P. Effects of statins on high-density lipoproteins: a potential contribution to cardiovascular benefit. Cardiovasc Drugs Ther. 2008; 22:321–38.

4. van Duijnhoven FJ, Bueno-De-Mesquita HB, Calligaro M, Jenab M, Pischon T, Jansen EH, et al. Blood lipid and lipoprotein concentrations and colorectal cancer risk in the European Prospective Investigation into Cancer and Nutrition. Gut. 2011; 60:1094–102.
5. Ahn J, Lim U, Weinstein SJ, Schatzkin A, Hayes RB, Virtamo J, et al. Prediagnostic total and high-density lipoprotein cholesterol and risk of cancer. Cancer Epidemiol Biomarkers Prev. 2009; 18:2814–21.

6. Pedersen KM, Colak Y, Bojesen SE, Nordestgaard BG. Low high-density lipoprotein and increased risk of several cancers: 2 population-based cohort studies including 116,728 individuals. J Hematol Oncol. 2020; 13:129.

7. Furberg AS, Veierod MB, Wilsgaard T, Bernstein L, Thune I. Serum high-density lipoprotein cholesterol, metabolic profile, and breast cancer risk. J Natl Cancer Inst. 2004; 96:1152–60.

8. Kucharska-Newton AM, Rosamond WD, Schroeder JC, McNeill AM, Coresh J, Folsom AR, et al. HDL-cholesterol and the incidence of lung cancer in the Atherosclerosis Risk in Communities (ARIC) study. Lung Cancer. 2008; 61:292–300.

9. Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA. 2006; 295:2164–7.

10. Kim TY, Kim WG, Kim WB, Shong YK. Current status and future perspectives in differentiated thyroid cancer. Endocrinol Metab (Seoul). 2014; 29:217–25.

11. Morris LG, Myssiorek D. Improved detection does not fully explain the rising incidence of well-differentiated thyroid cancer: a population-based analysis. Am J Surg. 2010; 200:454–61.

12. Marcello MA, Cunha LL, Batista FA, Ward LS. Obesity and thyroid cancer. Endocr Relat Cancer. 2014; 21:T255–71.

13. Xu L, Port M, Landi S, Gemignani F, Cipollini M, Elisei R, et al. Obesity and the risk of papillary thyroid cancer: a pooled analysis of three case-control studies. Thyroid. 2014; 24:966–74.

14. Fussey JM, Beaumont RN, Wood AR, Vaidya B, Smith J, Tyrrell J. Does obesity cause thyroid cancer? A Mendelian randomization study. J Clin Endocrinol Metab. 2020; 105:e2398–407.

15. Kitahara CM, Platz EA, Freeman LE, Hsing AW, Linet MS, Park Y, et al. Obesity and thyroid cancer risk among U.S. men and women: a pooled analysis of five prospective studies. Cancer Epidemiol Biomarkers Prev. 2011; 20:464–72.

16. Kwon H, Han KD, Park CY. Weight change is significantly associated with risk of thyroid cancer: a nationwide population-based cohort study. Sci Rep. 2019; 9:1546.

17. Park JH, Choi M, Kim JH, Kim J, Han K, Kim B, et al. Metabolic syndrome and the risk of thyroid cancer: a nationwide population-based cohort study. Thyroid. 2020; 30:1496–504.

18. Lee J, Lee JS, Park SH, Shin SA, Kim K. Cohort profile: the National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int J Epidemiol. 2017; 46:e15.

19. Ahn HY, Park YJ. Incidence and clinical characteristics of thyroid cancer in Korea. Korean J Med. 2009; 77:537–42.
20. Rossi ED, Pantanowitz L, Hornick JL. A worldwide journey of thyroid cancer incidence centred on tumour histology. Lancet Diabetes Endocrinol. 2021; 9:193–4.

21. Ayturk S, Gursoy A, Kut A, Anil C, Nar A, Tutuncu NB. Metabolic syndrome and its components are associated with increased thyroid volume and nodule prevalence in a mildto-moderate iodine-deficient area. Eur J Endocrinol. 2009; 161:599–605.

22. Rojeski MT, Gharib H. Nodular thyroid disease. Evaluation and management. N Engl J Med. 1985; 313:428–36.
23. Li D, Zhou L, Ma C, Chen W, Zhang Y, Yu S, et al. Comparative analysis of the serum proteome profiles of thyroid cancer: an initial focus on the lipid profile. Oncol Lett. 2019; 18:3349–57.

24. Zhao W, Guan J, Horswell R, Li W, Wang Y, Wu X, et al. HDL cholesterol and cancer risk among patients with type 2 diabetes. Diabetes Care. 2014; 37:3196–203.

25. Kwon H, Chang Y, Cho A, Ahn J, Park SE, Park CY, et al. Metabolic obesity phenotypes and thyroid cancer risk: a cohort study. Thyroid. 2019; 29:349–58.

26. Jafri H, Alsheikh-Ali AA, Karas RH. Baseline and on-treatment high-density lipoprotein cholesterol and the risk of cancer in randomized controlled trials of lipid-altering therapy. J Am Coll Cardiol. 2010; 55:2846–54.

27. Barter PJ, Brandrup-Wognsen G, Palmer MK, Nicholls SJ. Effect of statins on HDL-C: a complex process unrelated to changes in LDL-C. Analysis of the VOYAGER Database. J Lipid Res. 2010; 51:1546–53.

28. Rashid S, Uffelman KD, Lewis GF. The mechanism of HDL lowering in hypertriglyceridemic, insulin-resistant states. J Diabetes Complications. 2002; 16:24–8.

29. Aschebrook-Kilfoy B, Sabra MM, Brenner A, Moore SC, Ron E, Schatzkin A, et al. Diabetes and thyroid cancer risk in the National Institutes of Health-AARP Diet and Health Study. Thyroid. 2011; 21:957–63.

30. Fox CS, Pencina MJ, D’Agostino RB, Murabito JM, Seely EW, Pearce EN, et al. Relations of thyroid function to body weight: cross-sectional and longitudinal observations in a community-based sample. Arch Intern Med. 2008; 168:587–92.

31. Iacobellis G, Ribaudo MC, Zappaterreno A, Iannucci CV, Leonetti F. Relationship of thyroid function with body mass index, leptin, insulin sensitivity and adiponectin in euthyroid obese women. Clin Endocrinol (Oxf). 2005; 62:487–91.

32. Kim HJ, Park SJ, Park HK, Byun DW, Suh K, Yoo MH. Thyroid autoimmunity and metabolic syndrome: a nationwide population-based study. Eur J Endocrinol. 2021; 185:707–15.

33. Bjoro T, Holmen J, Kruger O, Midthjell K, Hunstad K, Schreiner T, et al. Prevalence of thyroid disease, thyroid dysfunction and thyroid peroxidase antibodies in a large, unselected population. The Health Study of Nord-Trondelag (HUNT). Eur J Endocrinol. 2000; 143:639–47.

34. Lacroix L, Pourcher T, Magnon C, Bellon N, Talbot M, Intaraphairot T, et al. Expression of the apical iodide transporter in human thyroid tissues: a comparison study with other iodide transporters. J Clin Endocrinol Metab. 2004; 89:1423–8.

35. Ichikawa Y, Saito E, Abe Y, Homma M, Muraki T. Presence of TSH receptor in thyroid neoplasms. J Clin Endocrinol Metab. 1976; 42:395–8.

36. Fiore E, Rago T, Provenzale MA, Scutari M, Ugolini C, Basolo F, et al. Lower levels of TSH are associated with a lower risk of papillary thyroid cancer in patients with thyroid nodular disease: thyroid autonomy may play a protective role. Endocr Relat Cancer. 2009; 16:1251–60.

37. Duntas LH, Brenta G. A renewed focus on the association between thyroid hormones and lipid metabolism. Front Endocrinol (Lausanne). 2018; 9:511.

38. Willard DL, Leung AM, Pearce EN. Thyroid function testing in patients with newly diagnosed hyperlipidemia. JAMA Intern Med. 2014; 174:287–9.

39. Vekic J, Kotur-Stevuljevic J, Jelic-Ivanovic Z, Spasic S, Spasojevic- Kalimanovska V, Topic A, et al. Association of oxidative stress and PON1 with LDL and HDL particle size in middle-aged subjects. Eur J Clin Invest. 2007; 37:715–23.

40. Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, Han S, et al. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science. 2010; 328:1689–93.

Fig. 2.
Definition of groups for the statistical analysis. HDL-C, high-density lipoprotein cholesterol.
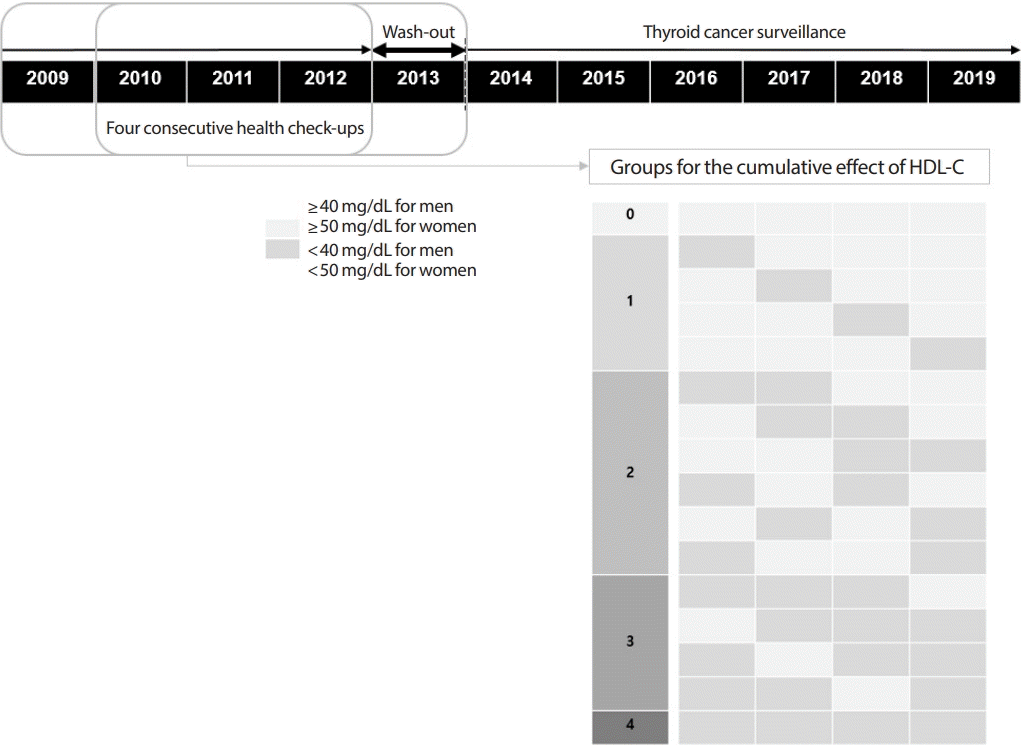
Fig. 3.
The cumulative effect of low high-density lipoprotein cholesterol levels on the incidence of thyroid cancer.
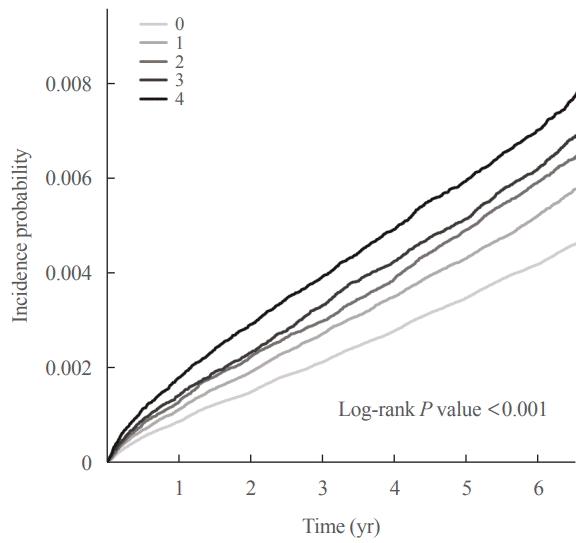
Table 1.
Baseline Characteristics of the Study Population
Table 2.
Low High-Density Lipoprotein Cholesterol Levels and the Risk of Thyroid Cancer
Multivariable analysis; Model 1, non-adjusted; Model 2, adjusted age, sex; Model 3, adjusted age, sex, smoking, drinking, regular exercise, income status; Model 4, adjusted age, sex, smoking, drinking, regular exercise, income status, waist circumference, high blood pressure, dyslipidemia, diabetes.
IR, incidence rate; HR, hazard ratio; CI, confidence interval.
Table 3.
Effects of Low High-Density Lipoprotein Cholesterol Levels on Thyroid Cancer According to Metabolic Status
| Subgroup | Group | Total no. | Patients no. | IR per 1,000 | HRa (95% CI) | P for interaction |
|---|---|---|---|---|---|---|
| Abdominal obesityb | 0.062 | |||||
| No | 0 | 1,580,957 | 6,833 | 0.695 | 1 (reference) | |
| 1 | 427,869 | 2,300 | 0.865 | 1.145 (1.092–1.201) | ||
| 2 | 239,096 | 1,467 | 0.988 | 1.275 (1.205–1.350) | ||
| 3 | 174,546 | 1,141 | 1.052 | 1.323 (1.242–1.410) | ||
| 4 | 166,879 | 1,212 | 1.170 | 1.459 (1.370–1.553) | ||
| Yes | 0 | 234,145 | 1,158 | 0.801 | 1 (reference) | |
| 1 | 103,174 | 601 | 0.944 | 1.154 (1.046–1.275) | ||
| 2 | 72,762 | 457 | 1.018 | 1.220 (1.094–1.361) | ||
| 3 | 61,997 | 404 | 1.055 | 1.241 (1.106–1.392) | ||
| 4 | 72,853 | 556 | 1.238 | 1.460 (1.315–1.621) | ||
| High blood pressure | 0.057 | |||||
| No | 0 | 1,540,448 | 6,900 | 0.780 | 1 (reference) | |
| 1 | 419,759 | 2,309 | 0.885 | 1.144 (1.091–1.199) | ||
| 2 | 231,212 | 1,476 | 1.027 | 1.298 (1.226–1.374) | ||
| 3 | 164,617 | 1,105 | 1.079 | 1.332 (1.249–1.420) | ||
| 4 | 139,848 | 1,106 | 1.271 | 1.536 (1.440–1.639) | ||
| Yes | 0 | 274,654 | 1,091 | 0.643 | 1 (reference) | |
| 1 | 111,284 | 592 | 0.863 | 1.280 (1.157–1.415) | ||
| 2 | 80,646 | 448 | 0.901 | 1.301 (1.164–1.454) | ||
| 3 | 71,926 | 440 | 0.992 | 1.397 (1.248–1.564) | ||
| 4 | 99,884 | 662 | 1.076 | 1.524 (1.378–1.684) | ||
| Impaired fasting glucosec | 0.051 | |||||
| No | 0 | 1,342,171 | 6,299 | 0.754 | 1 (reference) | |
| 1 | 369,558 | 2,112 | 0.919 | 1.135 (1.080–1.193) | ||
| 2 | 206,464 | 1,350 | 1.051 | 1.270 (1.197–1.348) | ||
| 3 | 149,306 | 1,064 | 1.145 | 1.354 (1.267–1.446) | ||
| 4 | 133,939 | 1,105 | 1.326 | 1.547 (1.449–1.651) | ||
| Yes | 0 | 472,931 | 1,692 | 0.578 | 1 (reference) | |
| 1 | 161,485 | 789 | 0.792 | 1.299 (1.193–1.414) | ||
| 2 | 105,394 | 574 | 0.882 | 1.393 (1.256–1.544) | ||
| 3 | 87,237 | 481 | 0.893 | 1.420 (1.290–1.562) | ||
| 4 | 105,793 | 663 | 1.017 | 1.572 (1.432–1.725) | ||
| Hyperlipidemia | 0.126 | |||||
| No | 0 | 1,638,520 | 7,281 | 0.715 | 1 (reference) | |
| 1 | 437,491 | 2,419 | 0.891 | 1.158 (1.105–1.213) | ||
| 2 | 239,499 | 1,526 | 1.027 | 1.304 (1.234–1.379) | ||
| 3 | 169,809 | 1,142 | 1.083 | 1.342 (1.260–1.429) | ||
| 4 | 133,886 | 1,115 | 1.340 | 1.595 (1.496–1.700) | ||
| Yes | 0 | 176,582 | 710 | 0.649 | 1 (reference) | |
| 1 | 93,552 | 482 | 0.832 | 1.326 (1.180–1.490) | ||
| 2 | 72,359 | 398 | 0.889 | 1.436 (1.267–1.628) | ||
| 3 | 66,734 | 403 | 0.976 | 1.582 (1.394–1.796) | ||
| 4 | 105,846 | 653 | 1.000 | 1.714 (1.529–1.921) |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download