Abstract
Background
Obesity, the prevalence of which is increasing due to the lack of exercise and increased consumption of Westernized diets, induces various complications, including ophthalmic diseases. For example, obesity is involved in the onset of cataracts.
Methods
To clarify the effects and mechanisms of midodrine, an α1-adrenergic receptor agonist, in cataracts induced by obesity, we conducted various analytic experiments in Otsuka Long-Evans Tokushima Fatty (OLETF) rats, a rat model of obesity.
Results
Midodrine prevented cataract occurrence and improved lens clearance in OLETF rats. In the lenses of OLETF rats treated with midodrine, we observed lower levels of aldose reductase, tumor necrosis factor-α, and sorbitol, but higher levels of hexokinase, 5’-adenosine monophosphate-activated protein kinase-alpha, adenosine 5´-triphosphate, peroxisome proliferator-activated receptor-delta, peroxisome proliferator-activated receptor gamma coactivator 1-alpha, superoxide dismutase, and catalase.
Conclusion
The ameliorating effects of midodrine on cataracts in the OLETF obesity rat model are exerted via the following three mechanisms: direct inhibition of the biosynthesis of sorbitol, which causes cataracts; reduction of reactive oxygen species and inflammation; and (3) stimulation of normal aerobic glycolysis.
Obesity is rapidly increasing worldwide due to a lack of physical activity and increased consumption of Westernized diets. According to a World Health Organization report, 39% and 13% of adults aged 18 years and over were overweight and obese, respectively, in 2016. Furthermore, the worldwide prevalence of obesity nearly tripled from 1975 to 2016 [1]. Obesity can cause several metabolic diseases such as hypertension, cardiovascular disease, and diabetes [2-4]. In addition, many reports have strongly suggested that obesity and dyslipidemia, which is characterized by high triglyceride (TG), high low-density lipoprotein (LDL)-cholesterol, and low high-density lipoprotein (HDL)-cholesterol levels, are important risk factors for cataracts. Furthermore, the correlation between obesity and cataracts may be higher in aged patients [5-7]. Cataracts, in which clouding of the eye lens obstructs visual function, are one of the main causes of blindness globally [8]. Cataracts are caused by aging, inflammation, oxidative stress, radiation, and particularly diabetes and obesity [5-7,9]. According to epidemiological studies, cataracts are the most common cause of visual impairment in patients with late-onset diabetes [10,11]. Ophthalmic surgery is the only available treatment for cataracts. Therefore, a common remedy for obesity and cataracts would be a major milestone that would offer a long-sought treatment option for cataract patients both with and without accompanying obesity.
Midodrine (2-amino-N-[2-(2,5-dimethoxyphenyl)-2-hydroxy-ethyl]-acetamide) is used to treat orthostatic hypotension and dysautonomia. In the body, it is converted by a peptidase to its active form, desglymidodrine, which binds to the α1-adrenergic receptor in blood vessels and induces blood pressure (BP) elevation [12].
According to a recent study, dabuzalgron, an α1-adrenergic receptor agonist, inhibits the cardiotoxicity of doxorubicin by activating genes related to mitochondrial function and energy production [13]. Additionally, metabolic diseases such as diabetes and obesity are characterized by reduced catabolic metabolism, and as a result, lowered adenosine 5´-triphosphate (ATP) production. Therefore, we can speculate that midodrine, an α1-adrenergic receptor agonist, might have similar effects and ameliorate both obesity and its complications, such as cataracts.
The aim of this study was to establish the effects and mechanisms of action of midodrine on early-stage cataracts in Otsuka Long-Evans Tokushima Fatty (OLETF) rats, an obesity model. To this end, we studied the effects and functions of midodrine on various biomarkers related to the occurrence and regulation of cataracts in a rat model of obesity.
Male OLETF rats and Long-Evans Tokushima Otsuka (LETO) rats (normal nondiabetic counterparts) were obtained from Doo Yeol Biotech (Seoul, Korea). A mydriasis reagent (Mydrin-P) was obtained from Santen (Osaka, Japan). Enzyme-linked immunosorbent assay (ELISA) kits for aldose reductase (AR), sorbitol dehydrogenase (SD), catalase, sorbitol, hexokinase, ATP, and tumor necrosis factor-alpha (TNFα) were purchased from Mybiosource (San Diego, CA, USA). ELISA kits for TG and cholesterol were obtained from Abcam (Cambridge, UK). An ELISA kit for 5’-adenosine monophosphate-activated protein kinase alpha (AMPKα) was bought from Invitrogen (Carlsbad, CA, USA). A protein-extracting reagent was purchased from Intron Biotechnology (Seongnam, Korea). Primary antibodies for peroxisome proliferator-activated receptor delta (PPARδ) and peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) were obtained from Abcam. The primary antibody for superoxide dismutase (SOD) was supplied from Novus Biologicals (Littleton, CO, USA). The primary antibody for β-actin was purchased from Santa Cruz Biotechnology Inc. (Dallas, TX, USA). Secondary antibodies were obtained from Vector Laboratories Inc. (Burlingame, CA, USA). All other reagents used in Western blotting and ELISA were purchased from Sigma-Aldrich (St. Louis, MO, USA). Kodak GBX developer and fixer reagents were purchased from Carestream Health Inc. (Rochester, NY, USA).
Besides a normal control group (six LETO rats) and a disease control group (six OLETF rats), there were two drug-treated groups (0.3 or 1.0 mg/kg/day of midodrine; six OLETF rats per group). The midodrine dose of 0.3 mg/kg/day (1.2 μM/kg/day) is equivalent to approximately 0.05 mg/kg/day, which does not increase BP in humans, and the midodrine concentration of 1.0 mg/kg/day is equivalent to the dose that increases BP levels in human clinical practice. Therefore, these two doses were used to compare the effects of the two concentrations in this study.
All animals were fed a normal control diet for 12 weeks, and the drug-treated groups were provided midodrine in their drinking water for 12 subsequent weeks. Four-week-old animals were acclimated for 7 days, and after induction of diabetes for 22 weeks, the animals aged 26 weeks were administered the drug for 12 weeks.
All rats were sacrificed in the morning of the same day. All animal experiments were conducted in accordance with the Animal Experiment Ethics Guide of Guro Hospital, Korea University, and complied with the Korea University Animal Science Rules and Regulations. The protocols were approved by the Korea University Institutional Animal Care and Use Committee (approval number: KOREA-2017-0020).
Fasting blood glucose levels were measured once a week as follows: food was removed from the cages in the morning, and blood glucose levels were measured using tail vein blood samples after 5 hours using a blood glucose meter (Roche, Basel, Switzerland). An oral glucose tolerance test (OGTT) was conducted at the start and end of the experiment. Glucose was administered to rats via oral gavage (2 g/kg), blood samples were collected from their tail veins 0, 30, 60, 90, and 120 minutes later, and blood glucose levels were measured.
After the animals were anesthetized with an intramuscular injection of alfaxalone (15 mg/kg) and Rompun (9.33 mg/kg), the mydriasis reagent (Mydrin-P), consisting of 0.5% tropicamide and 0.5% phenylephrine chloride, was administered into the eyes three times at a 10-minute interval to dilate the pupils. After dilating the pupils, retro-illumination photographs were taken with a handheld fundus camera (Horus Scope DEC 200, Jed Med, St. Louis, MO, USA) to obtain a sharply focused image of cortical and posterior subcapsular cataracts in each eye. The cataract grade (cortical and posterior capsular opacity) was assessed on the basis of the Lens Opacities Classification System III (LOCS III) in the retro-illumination photographs.
To evaluate nuclear sclerosis, which refers to cloudiness and hardening of the lens that reduces its transparency, the lens tissues were obtained from animals euthanized with anesthetic, and the extracted lenses were put on graph paper and photographed. The transparency of the lenses was analyzed with the ImageJ program.
The concentrations of TG, HDL-cholesterol, LDL-cholesterol, TNFα, and sorbitol in serum samples were estimated according to the manufacturer’s guidance. Lens tissues were rinsed in icecold phosphate-buffered saline (PBS) to remove impurities. Minced lens tissue (50 mg) was homogenized in 500 µL of PBS. The extract was centrifuged at 1,500 ×g for 15 minutes at 4°C, and the supernatant was transferred to another tube. The concentrations of AR, SD, hexokinase, and ATP in the lens extracts were measured according to the manufacturer’s instructions. To estimate the concentrations of AMPKα and catalase in eye tissues, such as the pupils, iris, and cornea, the eye tissue samples other than the lenses were rinsed in ice-cold PBS to remove impurities. Minced eye tissue (50 mg) was homogenized in 500 µL of protein-extracting solution. The extract was centrifuged at 13,000 rpm for 5 minutes at 4°C, and the supernatant was transferred to another tube. The concentrations of AMPKα, catalase, and sorbitol in eye tissues other than the lenses were estimated according to the manufacturer’s method. ELISA protocols were conducted with reference to a previously published paper [14].
The tissues were homogenized in protein-extracting reagent (Welgene Inc., Daegu, Korea) and the protein concentration of tissue extracts was estimated using the Bradford method. For each sample, 10 μg of the extracted proteins was loaded onto 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels. The proteins were transferred to nitrocellulose membranes using electroblotting for 90 minutes at 100 V, and non-specific binding to the membranes was blocked by overnight incubation of the membrane in 5% skim milk solution. After three 10-minute washes in Tris-buffered saline containing 0.05% Tween 20 (TBS-T), the membranes were incubated with solutions of primary antibodies at room temperature (25°C) for 2 hours. The following primary antibodies were used at 1:1,000 dilution: PPARδ, PGC-1α, SOD, and β-actin. After three further 10-minute washes in TBS-T, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies at room temperature for 1 hour. The following dilutions were used for the secondary antibodies: 1:10,000 for anti-rabbit immunoglobulin G (IgG) antibodies for PPARδ, PGC-1α, and SOD; and 1:10,000 for anti-mouse IgG antibody for β-actin. Subsequently, the membranes were washed three times in TBS-T for 10 minutes, once in TBS for 10 minutes, and then treated with chemiluminescent substrate and enhancer solutions. The images were obtained manually using developer and fixer reagents, and the results were analyzed using the ImageJ program. We followed the methods of Lee et al. [15].
The experimental results are presented as the mean±standard error of the mean. Statistically significant differences between two groups were calculated by the unpaired t test, and one-way analysis of variance was used to examine the statistical significance of differences among three or more groups. P values<0.05 were considered to indicate statistically significant differences. We followed the statistical method described by Han et al. [14].
Midodrine treatment (0.3 and 1.0 mg/kg/day) for 12 weeks reduced visceral fat weight and body weight compared to those of non-treated OLETF control rats (Fig. 1A, B). The 0.3 mg/kg/day dose showed a greater tendency than the dose of 1.0 mg/kg/day to reduce visceral fat weight and body weight.
The levels of TG and LDL-cholesterol were higher in the non-treated OLETF control group than in LETO rats. However, only the level of TG was decreased by midodrine treatment (Fig. 1C, E). Further, treatment with a midodrine dose of 0.3 mg/kg/day significantly increased HDL-cholesterol levels compared to those in non-treated OLETF controls (Fig. 1D). The 0.3 mg/kg/day dose elevated HDL-cholesterol levels more significantly than the 1.0 mg/kg/day concentration, and also resulted in a greater reduction of LDL levels than the 1.0 mg/kg/day dose. Midodrine did not significantly change fasting blood glucose levels and OGTT results (Fig. 1F-H).
In the LETO group (normal control rats), cataracts were not observed. However, in the non-treated OLETF control group, there was a high incidence of cataracts (66.7%; 8 of 12 eyes). This rate decreased to 10.0% (1 of 10 eyes) and 25% (2 of 8 eyes) after treatment with 0.3 and 1.0 mg/kg/day midodrine, respectively. The mean cortical opacity in both the LETO (0.0±0.0) and midodrine 0.3 mg/kg/day (0.2±0.6) groups was significantly lower than that in the non-treated OLETF control group (1.3±1.2) (P=0.004 and P=0.025, respectively). However, there was no significant difference in the mean cortical opacity between the non-treated OLETF control and midodrine 1.0 mg/kg/day (0.6±1.2) groups. The mean posterior capsular opacity in the non-treated OLETF control group (0.7±1.0) was significantly higher than that in the LETO (0.0±0.0), midodrine 0.3 mg/kg/day (0.0±0.0), and midodrine 1.0 mg/kg/day (0.0±0.0) groups, (P=0.019, P=0.027, and P=0.042, respectively) (Fig. 2A). Lens transparency in the non-treated OLETF control group was lower than that in the LETO group, but it improved with 0.3 mg/kg/day midodrine treatment. Treatment with 1.0 mg/kg/day midodrine showed only a non-significant trend toward improvement (Fig. 2B).
The concentration of AR, a key enzyme in the polyol pathway that induces cataracts, was higher in the lenses of non-treated OLETF rats than in both LETO rats and midodrine-treated OLETF rats (Fig. 3A). The level of SD, which converts sorbitol to fructose, was lower in non-treated OLETF rats than in LETO rats, but an increase was not observed in midodrine-treated rats (Fig. 3B). The level of hexokinase, the rate-limiting enzyme of glycolysis that converts glucose to glucose 6-phosphate, was lower in the lenses of non-treated OLETF rats than in LETO rats. However, the level was higher in OLETF rats treated with 1.0 mg/kg/day midodrine (Fig. 3C). The phosphorylated AMPK (p-AMPK) concentration in eye tissues except for lens was elevated in OLETF rats treated with 0.3 mg/kg/day midodrine compared to that in non-treated OLETF rats, and the 0.3 mg/kg/day dose significantly increased p-AMPK expression to a greater extent than the 1.0 kg/kg/day concentration (Fig. 3D). The concentration of ATP indicates the energy state in tissues or cells. In this study, both midodrine treatments elevated ATP levels in lenses compared to the levels in non-treated OLETF rats (Fig. 3E). The TNFα level was higher in serum samples of non-treated OLETF rats than in LETO rats; however, it was lower in OLETF rats treated with midodrine doses of 0.3 and 1.0 mg/kg/day (Fig. 3F). The concentration of catalase was lower in eye tissues, except for the lens, in non-treated OLETF rats than in LETO rats; however, the level was higher in OLETF rats treated with midodrine of 0.3 and 1.0 mg/kg/day (Fig. 3G). The level of sorbitol was higher in serum samples of non-treated OLETF rats than in LETO rats; however, it was lower in OLETF rats treated with a midodrine dose of 1.0 mg/kg/day (Fig. 3H).
The protein levels for PPARδ, PGC-1α, and SOD in eye tissues other than the lens were higher in OLETF rats treated with midodrine doses of 0.3 and 1.0 mg/kg/day than in non-treated OLETF rats, and PPARδ protein expression was more significantly increased in the group that received the 1.0 mg/kg/day dose than in the group that received the 0.3 mg/kg/day concentration (Fig. 3I).
In the general polyol pathway, glucose is converted to sorbitol by AR and sorbitol is changed to fructose by SD. However, in lenses with diabetic cataracts, excessive concentrations of glucose are metabolized to sorbitol, which accumulates and causes an increase in osmotic pressure leading to lens fiber damage and an increase in lens opacity [16].
Obesity is a risk factor of type 2 diabetes because it induces insulin resistance [17], and it is involved in the generation of cataracts. Although a study asserted that there was no correlation between obesity and cataract formation [18], most epidemiological studies have strongly suggested an intimate correlation between obesity and cataracts [19,21]. Furthermore, another study reported a specific mechanism of obesity in the development of cataracts, according to which obesity increases the incidence of cataracts by stimulating glucose-induced sorbitol accumulation [7].
In addition to the abnormal polyol pathway, inflammation, reactive oxygen species (ROS), and low ATP levels in the lens contribute to the pathogenesis of cataracts. More specifically, serum levels of inflammatory cytokines, such as interleukin 6 (IL-6), IL 8, and TNFα, were found to be elevated in patients with diabetes both with and without cataracts compared to those in healthy individuals and cataract patients who did not have diabetes. Moreover, the concentrations of inflammatory cytokines, including TNFα, were elevated to a greater extent in patients with diabetes and cataracts than cataract patients without diabetes [22]. As is also the case with diabetes, obesity exhibits chronic low-level inflammation in which the levels of pro-inflammatory cytokines such as IL-6 and TNFα are elevated [23], and pro-inflammatory cytokines are primarily produced by adipose tissues and induce obesity-related complications [24,25].
Moreover, the anti-inflammation mechanism of midodrine has already been reported; specifically, midodrine was found to transform the inflamed M1 type of macrophage to the non-inflammatory M2 type [26].
High levels of ROS are generated in obesity and diabetes [27,28], inducing or aggravating cataracts [29,30]. However, ROS-scavenging enzymes, such as SOD, protect lens epithelial cells against oxidative stress [31,32]. In the representative ROS scavenging pathway, superoxide (O2−) is converted to hydrogen peroxide (H2O2) by SOD, and then the H2O2 is detoxified to H2O and O2 by catalase.
Furthermore, a decrease in the ATP concentration in the lens is correlated with the development of cataracts [33]. In general, the lens acquires 70% of its required energy (ATP) via anaerobic glycolysis, which accounts for 85% of glucose metabolized by the lens. However, lens epithelial cells support 20% of the energy required by the lens through aerobic glycolysis, which accounts for only 3% of glucose metabolized by the lens [34]. Because the energy production efficiency of aerobic glycolysis in the lens is relatively high despite the low glucose degradation rate, aerobic glycolysis has an important function in ATP production by the lens. Therefore, catabolic metabolism, which generates ATP, can contribute to the prevention and treatment of cataracts induced by diabetes and obesity.
For this reason, it is reasonable to argue that elevated inflammatory cytokine and ROS levels and reduced ATP levels play an important role in the pathogenesis of diabetes- and obesity-induced cataracts. The development of drugs capable of alleviating the effects of the abnormal polyol pathway, increased inflammation and ROS, and lowered energy state in eye cells could help cure diabetes- and obesity-related cataracts, as well as cataracts in general.
Catecholamines, such as epinephrine and norepinephrine, which are implicated in the fight-or-flight response to acute stress, suppress obesity through the activation of fatty acid oxidation [35]. Accordingly, it can be speculated that catecholamine receptors have the potential to be used as a target for diabetes- and obesity-related cataracts, particularly the α1-adrenergic receptor. Several studies have assessed the possibility of using the α1-adrenergic receptor as a target for various diseases. α1-Adrenergic receptor agonists such as phenylephrine and cirazoline stimulated glucose uptake in L6 rat myocytes [36]. Moreover, α1-adrenergic receptor agonists, such as dabuzalgron, improved the cardiotoxicity caused by doxorubicin through mitochondrial activation [13]. Another α1-adrenergic receptor agonist, A61603, decreased the levels of polyunsaturated fatty acids in the heart and endocannabinoid metabolites involved in inflammation [37]. From previous reports on the effects of the α1-adrenergic receptor on glucose utilization, mitochondrial function, and anti-inflammation, there is sufficient evidence to argue for the therapeutic efficacy of midodrine on cataracts induced by diabetes and obesity.
Generally, the degradation of glucose via oxidative phosphorylation is sequentially regulated by PPARδ, AMPK, and PGC-1α. In PPARδ null mice, the metabolic rate was generally reduced, and they exhibited insulin intolerance [38]. In one study, an injection of the AMPK activator, 5-aminoimidazole-4-carboxamide-1-β-D ribonucleoside, elevated hexokinase 2 mRNA levels in skeletal muscle tissues [39]. The over-expression of PGC-1α, a coactivator regulated by AMPK, improved oxidative phosphorylation in cells harboring deficient mitochondrial genes [40-42]. Furthermore, AMPK expression in several cell lines, such as human live cancer cell line (HepG2), human embryonic kidney 293 cell line (HEK293), human umbilical vein endothelial cells (HUVECs), and embryonic mouse fibroblasts 3T3-L1 cell line (3T3-L1), was regulated by PPARδ [15,43]. In addition, dyslipidemia is closely related to diabetic cataracts. The onset and aggravation of diabetic cataracts were positively correlated with TG levels, but negatively correlated with levels of HDL-cholesterol [44].
In our study, the levels of hexokinase, PPARδ, AMPK, and PGC-1α in eye tissues increased after midodrine treatment, and as the final product of aerobic and anaerobic glycolysis, ATP concentrations in lens tissues were higher in the midodrine-treated groups than in the OLETF disease control group. The activation of catabolic metabolism by midodrine may lead to improvement of dyslipidemia, including a decrease in TG and an increase in HDL-cholesterol levels, and may therefore reduce visceral fat and body weight. As a result, midodrine treatment may ameliorate lens opacity in rats with type 2 diabetes and obesity. Body and visceral fat weights were significantly reduced in the midodrine-treated groups compared to the OLETF disease control group in this study. However, midodrine did not improve the blood glucose level in this study. Therefore, it is inferred that the effects of midodrine on cataract amelioration are mainly exerted through its anti-obesity effects.
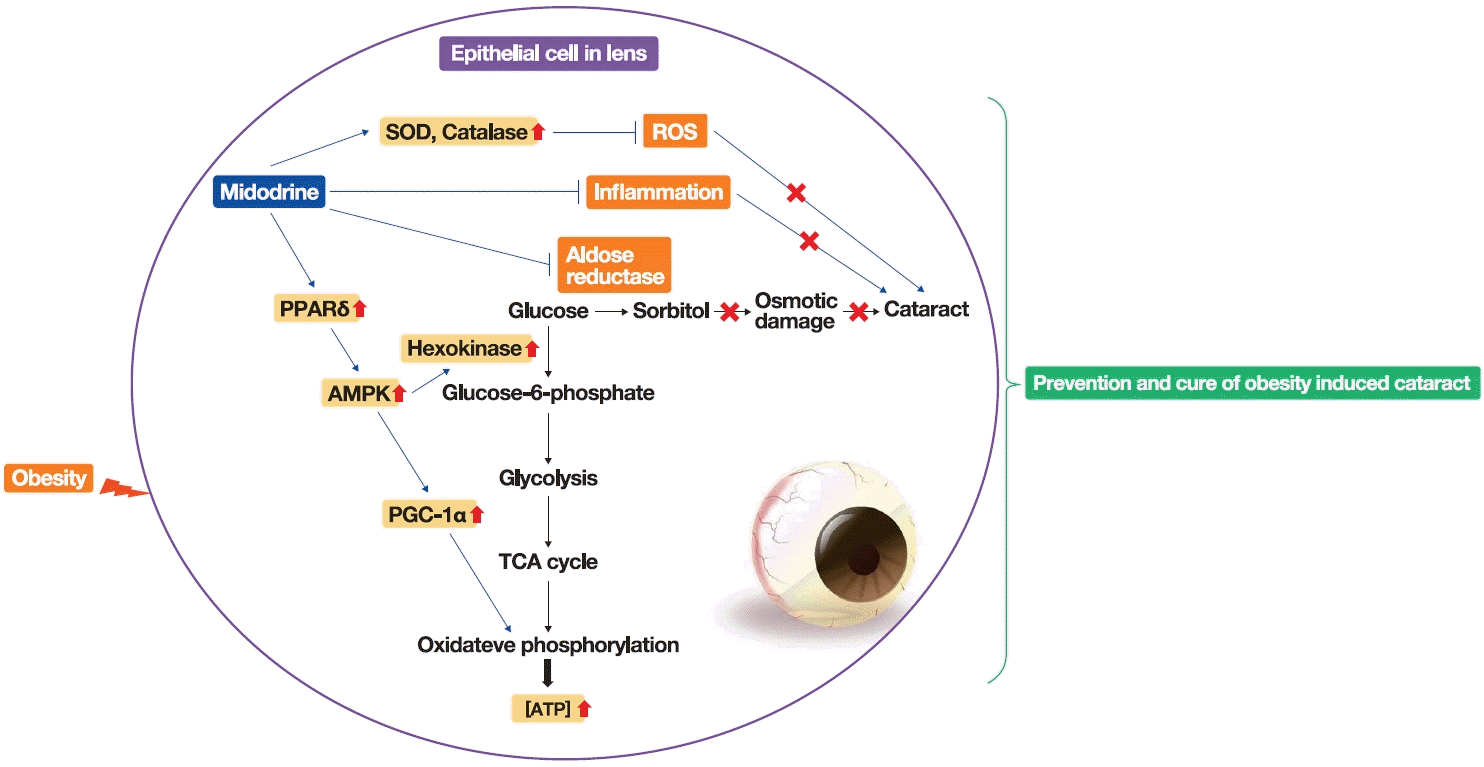
In conclusion, midodrine, an α1-adrenergic receptor agonist, can ameliorate early-stage cataracts induced by obesity through the following mechanisms: reduction of sorbitol synthesis via the inhibition of AR; alleviation of ROS by stimulating SOD and catalase; diminishment of inflammation by suppressing TNFα, and promotion of oxidative glycolysis through the activation of biomarkers involved in glucose degradation (Fig. 4). It is generally known that other metabolic factors, such as BP, also participate in the occurrence of cataracts. Therefore, further research exploring the correlation between obesity-induced cataracts and BP will be necessary.
To date, many drugs for lowering blood glucose levels and anti-obesity drugs have been developed and marketed, but drugs for treating general cataracts, as well as diabetes- and obesity-induced cataracts, are lacking. Our study may provide a small stepping stone toward the development of novel medicines for cataracts induced by diabetes and obesity.
Notes
ACKNOWLEDGMENTS
This research was supported by a grant from the National Research Foundation of Korea (NRF-2016R1A2B3013825); a grant from the Ministry of Future Creation and Science of Korea (2018K000255); a fund from Korea University Guro Hospital, Seoul, Republic of Korea; a grant from Korean Hypertension Management Association; a grant from BK21 Plus Korea University Medical Science graduate program; and the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Korean government (2021R1F1A1050004).
REFERENCES
1. World Health Organization. Obesity and overweight [Internet]. Geneva: WHO;2021. [cited 2022 Feb 22]. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
2. Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res. 2015; 116:991–1006.
3. Nakamura K, Fuster JJ, Walsh K. Adipokines: a link between obesity and cardiovascular disease. J Cardiol. 2014; 63:250–9.

4. Barnes AS. The epidemic of obesity and diabetes: trends and treatments. Tex Heart Inst J. 2011; 38:142–4.
5. Azim SF, Akhter QS, Akter T, Farid F. Obesity and dyslipidemia: risk factors for development of senile cataract. J Bangladesh Soc Physiol. 2018; 13:29–34.

6. Leske MC, Wu SY, Hennis A, Connell AM, Hyman L, Schachat A. Diabetes, hypertension, and central obesity as cataract risk factors in a black population: the Barbados Eye Study. Ophthalmology. 1999; 106:35–41.

7. Reddy PY, Giridharan NV, Balakrishna N, Validandi V, Pullakhandam R, Reddy GB. Increased risk of cataract development in WNIN-obese rats due to accumulation of intralenticular sorbitol. IUBMB Life. 2013; 65:472–8.

8. World Health Organization. Global data on visual impairments [Internet]. Geneva: WHO;2021. [cited 2022 Feb 22]. Available from: https://www.who.int/news-room/fact-sheets/detail/blindness-and-visual-impairment.
9. Klein BE, Klein R, Moss SE. Incidence of cataract surgery in the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Am J Ophthalmol. 1995; 119:295–300.

10. Klein BE, Klein R, Moss SE. Prevalence of cataracts in a population-based study of persons with diabetes mellitus. Ophthalmology. 1985; 92:1191–6.

12. Cruz DN. Midodrine: a selective alpha-adrenergic agonist for orthostatic hypotension and dialysis hypotension. Expert Opin Pharmacother. 2000; 1:835–40.
13. Beak J, Huang W, Parker JS, Hicks ST, Patterson C, Simpson PC, et al. An oral selective alpha-1A adrenergic receptor agonist prevents doxorubicin cardiotoxicity. JACC Basic Transl Sci. 2017; 2:39–53.

14. Han YM, Lee YJ, Jang YN, Kim HM, Seo HS, Jung TW, et al. Aspirin improves nonalcoholic fatty liver disease and atherosclerosis through regulation of the PPARδ-AMPKPGC-1α pathway in dyslipidemic conditions. Biomed Res Int. 2020; 2020:7806860.
15. Lee YJ, Jang YN, Han YM, Kim HM, Seo HS. 6-Gingerol normalizes the expression of biomarkers related to hypertension via PPARδ in HUVECs, HEK293, and differentiated 3T3-L1 cells. PPAR Res. 2018; 2018:6485064.
16. Kinoshita JH, Fukushi S, Kador P, Merola LO. Aldose reductase in diabetic complications of the eye. Metabolism. 1979; 28(4 Suppl 1):462–9.

17. Tataranni PA. Pathophysiology of obesity-induced insulin resistance and type 2 diabetes mellitus. Eur Rev Med Pharmacol Sci. 2002; 6:27–32.
18. Noran NH, Nooriah S, Mimiwati Z. The association between body mass index and age related cataract. Med J Malaysia. 2007; 62:49–52.
19. Weintraub JM, Willett WC, Rosner B, Colditz GA, Seddon JM, Hankinson SE. A prospective study of the relationship between body mass index and cataract extraction among US women and men. Int J Obes Relat Metab Disord. 2002; 26:1588–95.

20. Schaumberg DA, Glynn RJ, Christen WG, Hankinson SE, Hennekens CH. Relations of body fat distribution and height with cataract in men. Am J Clin Nutr. 2000; 72:1495–502.

21. Hiller R, Podgor MJ, Sperduto RD, Nowroozi L, Wilson PW, D’Agostino RB, et al. A longitudinal study of body mass index and lens opacities: the Framingham Studies. Ophthalmology. 1998; 105:1244–50.

22. Hamid S, Gul A, Hamid Q. Relationship of cytokines and AGE products in diabetic and non-diabetic patients with cataract. Int J Health Sci (Qassim). 2016; 10:507–15.

23. Lee H, Lee IS, Choue R. Obesity, inflammation and diet. Pediatr Gastroenterol Hepatol Nutr. 2013; 16:143–52.

24. Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003; 112:1821–30.

25. Ellulu MS, Patimah I, Khaza’ai H, Rahmat A, Abed Y. Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci. 2017; 13:851–63.

26. Lee YJ, Kim HS, Seo HS, Na JO, Jang YN, Han YM, et al. Stimulation of alpha1-adrenergic receptor ameliorates cellular functions of multiorgans beyond vasomotion through PPARδ. PPAR Res. 2020; 2020:3785137.
27. Fernandez-Sanchez A, Madrigal-Santillan E, Bautista M, Esquivel-Soto J, Morales-Gonzalez A, Esquivel-Chirino C, et al. Inflammation, oxidative stress, and obesity. Int J Mol Sci. 2011; 12:3117–32.

28. Nogueira-Machado JA, Chaves MM. From hyperglycemia to AGE-RAGE interaction on the cell surface: a dangerous metabolic route for diabetic patients. Expert Opin Ther Targets. 2008; 12:871–82.

30. Kaur J, Kukreja S, Kaur A, Malhotra N, Kaur R. The oxidative stress in cataract patients. J Clin Diagn Res. 2012; 6:1629–32.

31. Matsui H, Lin LR, Ho YS, Reddy VN. The effect of up- and downregulation of MnSOD enzyme on oxidative stress in human lens epithelial cells. Invest Ophthalmol Vis Sci. 2003; 44:3467–75.

32. Lin D, Barnett M, Grauer L, Robben J, Jewell A, Takemoto L, et al. Expression of superoxide dismutase in whole lens prevents cataract formation. Mol Vis. 2005; 11:853–8.
33. Sippel TO. Energy metabolism in the lens during development of galactose cataract in rats. Invest Ophthalmol. 1966; 5:576–82.
34. Wong TY. The ophthalmology examinations review. Singapore: World Scientific Publishing Company;2001. p. 4.

35. Ushiama S, Ishimaru Y, Narukawa M, Yoshioka M, Kozuka C, Watanabe N, et al. Catecholamines facilitate fuel expenditure and protect against obesity via a novel network of the gut-brain axis in transcription factor Skn-1-deficient mice. EBioMedicine. 2016; 8:60–71.

36. Hutchinson DS, Bengtsson T. alpha1A-adrenoceptors activate glucose uptake in L6 muscle cells through a phospholipase C-, phosphatidylinositol-3 kinase-, and atypical protein kinase C-dependent pathway. Endocrinology. 2005; 146:901–12.
37. Willis MS, Ilaiwy A, Montgomery MD, Simpson PC, Jensen BC. The alpha-1A adrenergic receptor agonist A61603 reduces cardiac polyunsaturated fatty acid and endocannabinoid metabolites associated with inflammation in vivo. Metabolomics. 2016; 12:155.

38. Lee CH, Olson P, Hevener A, Mehl I, Chong LW, Olefsky JM, et al. PPARdelta regulates glucose metabolism and insulin sensitivity. Proc Natl Acad Sci U S A. 2006; 103:3444–9.
39. Stoppani J, Hildebrandt AL, Sakamoto K, Cameron-Smith D, Goodyear LJ, Neufer PD. AMP-activated protein kinase activates transcription of the UCP3 and HKII genes in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2002; 283:E1239–48.
40. Srivastava S, Barrett JN, Moraes CT. PGC-1alpha/beta upregulation is associated with improved oxidative phosphorylation in cells harboring nonsense mtDNA mutations. Hum Mol Genet. 2007; 16:993–1005.

41. Wan Z, Root-McCaig J, Castellani L, Kemp BE, Steinberg GR, Wright DC. Evidence for the role of AMPK in regulating PGC-1 alpha expression and mitochondrial proteins in mouse epididymal adipose tissue. Obesity (Silver Spring). 2014; 22:730–8.

42. Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007; 104:12017–22.
43. Lee YJ, Jang YN, Han YM, Kim HM, Jeong JM, Son MJ, et al. Caffeoylquinic acid-rich extract of Aster glehni F. Schmidt ameliorates nonalcoholic fatty liver through the regulation of PPARδ and adiponectin in ApoE KO mice. PPAR Res. 2017; 2017:3912567.
Fig. 1.
Body weight, visceral fat weight, and levels of triglycerides, cholesterol, and glucose in blood from Long-Evans Tokushima Otsuka (LETO) and Otsuka Long-Evans Tokushima Fatty (OLETF) rats. (A, B) Body weight and visceral fat weight were higher in the non-treated OLETF control group than in the LETO group. However, they were lower in the midodrine-treated groups. (C) Triglyceride (TG) levels were higher in the non-treated OLETF controls than in the LETO group. However, the levels were lower in the midodrine-treated groups. (D) High-density lipoprotein (HDL)-cholesterol levels was higher in the midodrine-treated groups than in the non-treated OLETF control group. (E) Low-density lipoprotein (LDL)-cholesterol levels showed only a trend to be higher in the non-treated OLETF control and midodrine-treated groups than in the LETO group. However, there were statistically significant differences among the three OLETF groups. (F) Fasting blood glucose concentrations were higher in the OLETF control group than in the LETO group. However, there were no significant differences between the OLETF control and midodrine-treated groups. (G, H) The oral glucose tolerance test (OGTT) results were not significantly different between the OLETF control and midodrine-treated groups before and after the start of the animal experiment. Although the OGTT results before the experiment were not significantly different between the non-treated OLETF and midodrine-treated groups, the results at the end of the experiment showed a tendency for the midodrine-treated groups to have lower levels than the non-treated OLETF control group. The results are expressed as mean±standard error of the mean (n=5 or 6). Values were statistically analyzed using the unpaired t-test and one-way analysis of variance. All experiments were repeated three times. Mido 0.3, midodrine 0.3 mg/kg/day; Mido 1.0, midodrine 1.0 mg/kg/day. aP<0.001 vs. LETO; bP<0.05 vs. non-treated OLETF; cP<0.01 vs. non-treated OLETF; dP<0.001 vs. non-treated OLETF; eP<0.05 non-treated OLETF vs. Mido 0.3 and Mido 1.0; fP<0.01 non-treated OLETF vs. Mido 0.3 and Mido 1.0; gP<0.001 non-treated OLETF vs. Mido 0.3 and Mido 1.0; hP<0.05 vs. Mido 1.0.
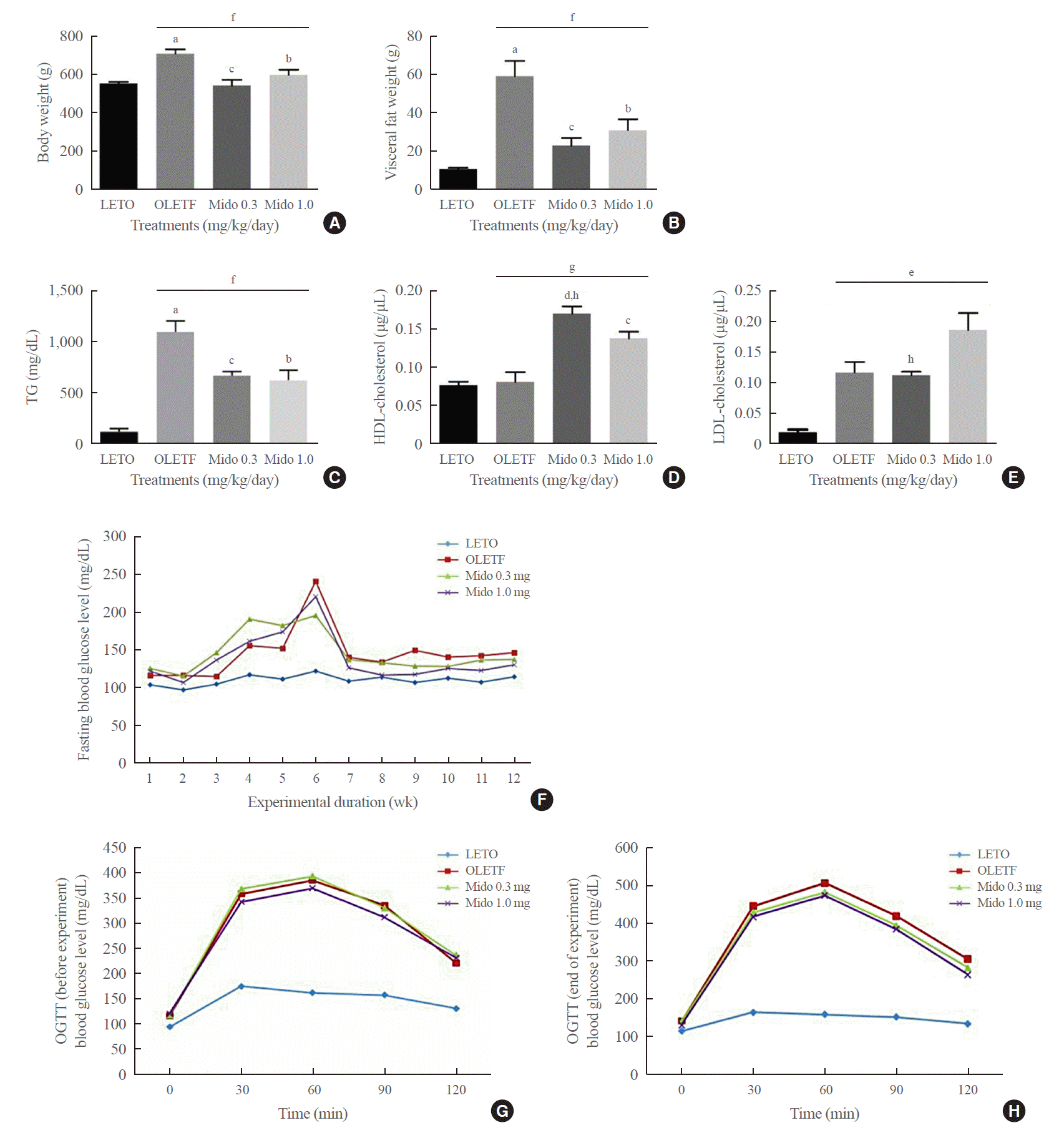
Fig. 2.
The occurrence rate of cataracts (ORC) and lens transparancy in Long-Evans Tokushima Otsuka (LETO) and Otsuka Long-Evans Tokushima Fatty (OLETF) rats. (A) The ORC and mean cortical and posterior opacities were higher in the non-treated OLETF control group than in the LETO normal control group; however, they were lower in the midodrine-treated groups. (B) Lens transparency was aggravated in the non-treated OLETF control group compared to that in the LETO normal control group. However, this change was ameliorated in the midodrine 0.3 mg/kg/day treatment group. The results are expressed as mean±standard error of the mean (n=8 to 12). Values were statistically analyzed using the unpaired t test and one-way analysis of variance. All experiments were repeated three times. aP<0.01 vs. LETO; bP<0.05 vs. non-treated OLETF.
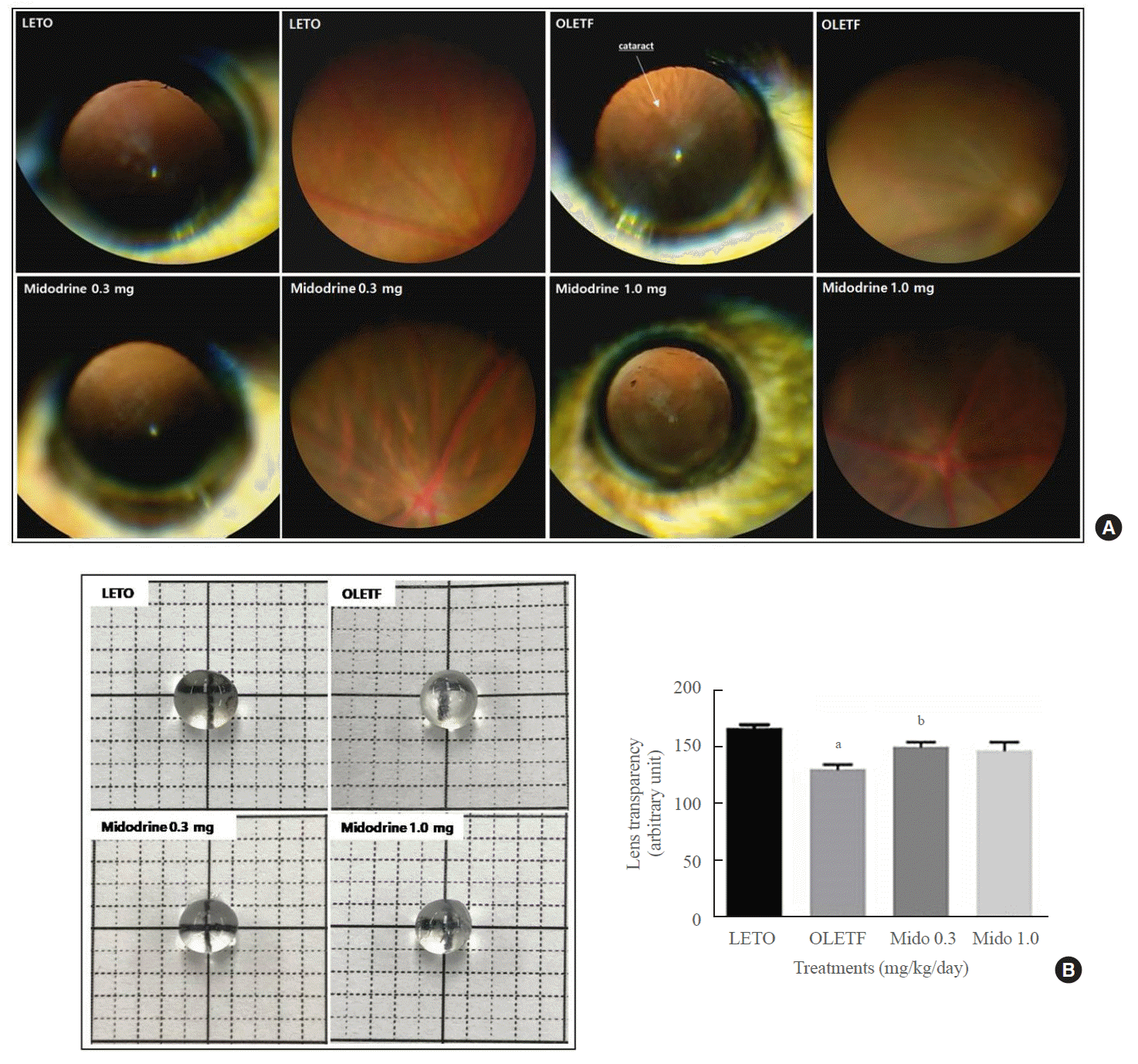
Fig. 3.
The concentrations of aldose reductase, sorbitol dehydrogenase, 5´-adenosine monophosphate-activated protein kinase alpha (AMPKα), hexokinase and adenosine 5´-triphosphate (ATP) in the lens, the concentration of tumor necrosis factor-α (TNFα) in serum, and the protein levels of peroxisome proliferator-activated receptor delta (PPARδ) and peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) in eye tissues other than the lens from Long-Evans Tokushima Otsuka (LETO) and Otsuka Long-Evans Tokushima Fatty (OLETF) rats. (A) The aldose reductase concentration in the lens was lower in the midodrine-treated groups than in the non-treated OLETF control group. (B) The decreased sorbitol dehydrogenase concentration in the lens in the non-treated OLETF control group was not changed by midodrine treatment. (C) The hexokinase concentration was lower in the lens in the non-treated OLETF control group than in the LETO normal control group, however, it was significantly higher in the midodrine 1.0 mg/kg/day treatment group. (D) The concentration of AMPKα (phosphorylated at the threonine 172 residue) in eye tissues other than the lens was significantly elevated in the midodrine 0.3 mg/kg/day treatment group. (E) The ATP concentration in the lens showed a tendency to be lower in the non-treated OLETF control than in the LETO normal control group; however, it was higher in the midodrine-treated groups. (F) The TNFα concentration in serum was higher in the non-treated OLETF control group than in the LETO normal control group; however, it was lower in the midodrine 0.3 mg/kg/day treatment group. (G) The catalase concentration in eye tissues other than the lens was lower in the non-treated OLETF control group than in the LETO normal control group; however, it was significantly higher in the midodrine-treated groups. (H) The sorbitol concentration in serum was higher in the non-treated OLETF control group than in the LETO normal control group; however, it was lower in midodrine 1.0 mg/kg/day treatment group. (I) The protein levels for PPARδ, PGC-1α, and superoxide dismutase (SOD) in eye tissues other than the lens were higher in the midodrine-treated groups. The results are expressed as mean±standard error of the mean (n=5 or 6). Values were statistically analyzed using the unpaired t test and one way analysis of variance (ANOVA). All experiments were repeated three times. Mido 0.3, midodrine 0.3 mg/kg/day; Mido 1.0, midodrine 1.0 mg/kg/day. aP<0.05 vs. LETO; bP<0.05 vs. non-treated OLETF; cP<0.01 vs. non-treated OLETF; dP<0.001 vs. non-treated OLETF; eP<0.05 non-treated OLETF vs. Mido 0.3 and Mido 1.0; fP<0.01 non-treated OLETF vs. Mido 0.3 and Mido 1.0; gP<0.001 non-treated OLETF vs. Mido 0.3 and Mido 1.0; hP<0.05 vs. Mido 1.0.
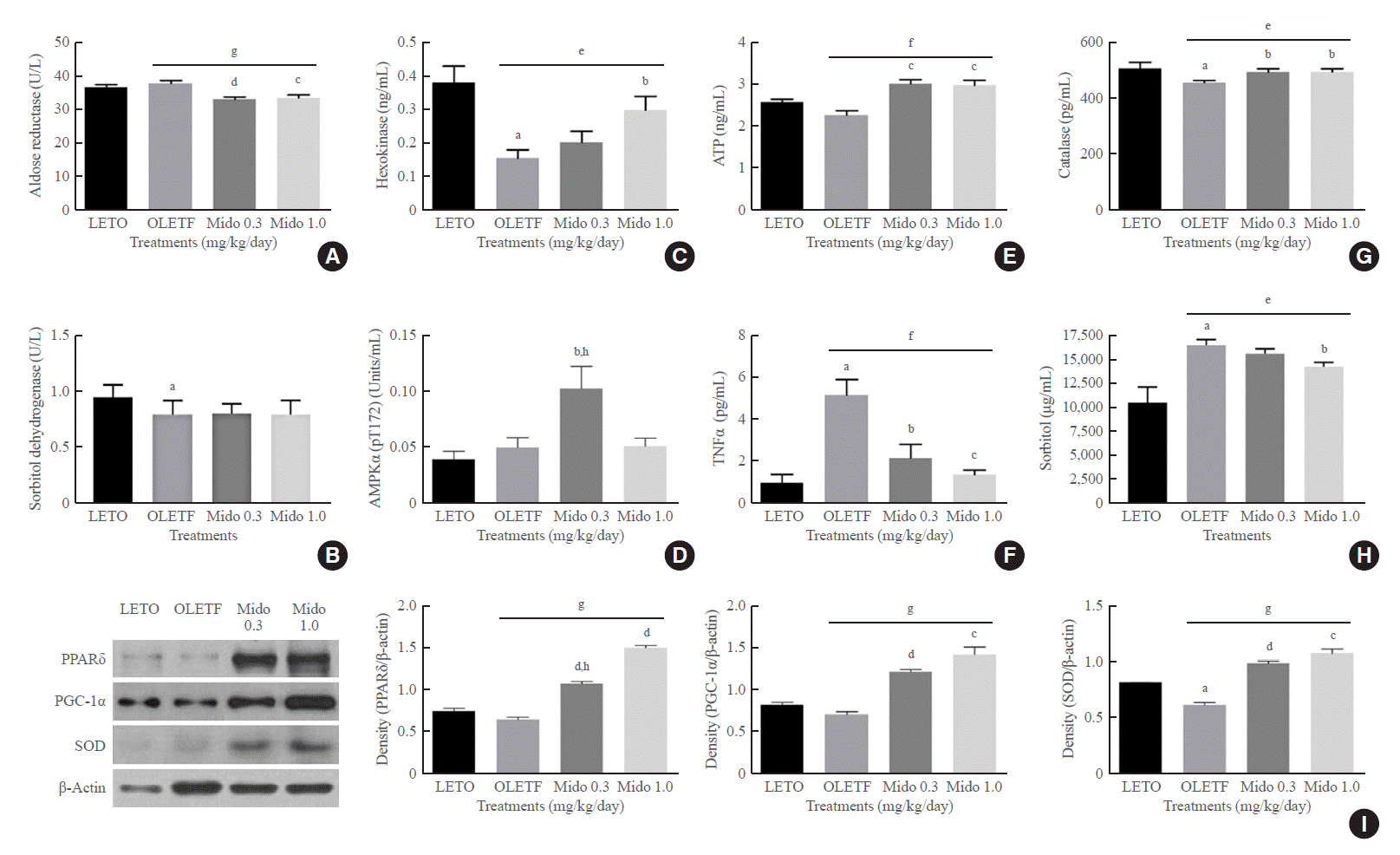
Fig. 4.
Diagram presenting the hypothesis generated in the present study for the effects and mechanism of midodrine on obesity-induced cataracts. Midodrine can prevent and cure obesity-induced cataracts through the inhibition of the polyol pathway, inflammation, and reactive oxygen species (ROS) and the activation of oxidative glycolysis. Meaning of symbols: A blue arrow indicates activation, blue upward and horizontal lines indicate inhibition, a red upward arrow indicates elevation, and a red X indicates blocking. PPARδ, peroxisome proliferator-activated receptor delta; AMPK, 5´-adenosine monophosphate-activated protein kinase; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; TCA, tricarboxylic acid cycle; ATP, adenosine 5´-triphosphate.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download