With the coronavirus disease 2019 (COVID-19) outbreak and the rise in vaccination rates, the interest in the therapeutic agents for COVID-19 has increased recently [1]. Although there is an urgent need to develop a powerful therapeutic agent for the suppression of COVID-19, the development of new drugs is actually a time-consuming and expensive task [2]. The success rate of new drug development is very low, with an average rate of 2.0%; hence, the risk of investing in new drug development is relatively high [3]. Notably, according to a report by the U.S. Food and Drug Administration (FDA), the number of drugs approved since 1995 has been steadily declining [4]. About 90% of the drugs do not pass the initial development and toxicity tests, whereas others that enter clinical trials later fail due to the incidence of side effects [5].
Amid these social issues, the interest in drug repositioning has increased. Drug repositioning involves changing the indications of the drugs already being used or those in development for the treatment of other diseases or exploring the possibility of new disease treatments [6]. In other words, it is to prove a new indication by finding a new relationship between an already marketed drug and a disease [7]. Therefore, application of drug repositioning in new drug development can significantly reduce the cost and time when compared with the traditional development of a new drug [8–10]. For example, both Victoza (Novo Nordisk, Bagsværd, Denmark) and Saxenda (Novo Nordisk) contain the same drug liraglutide, but the former is licensed for diabetes and the latter for obesity [11].
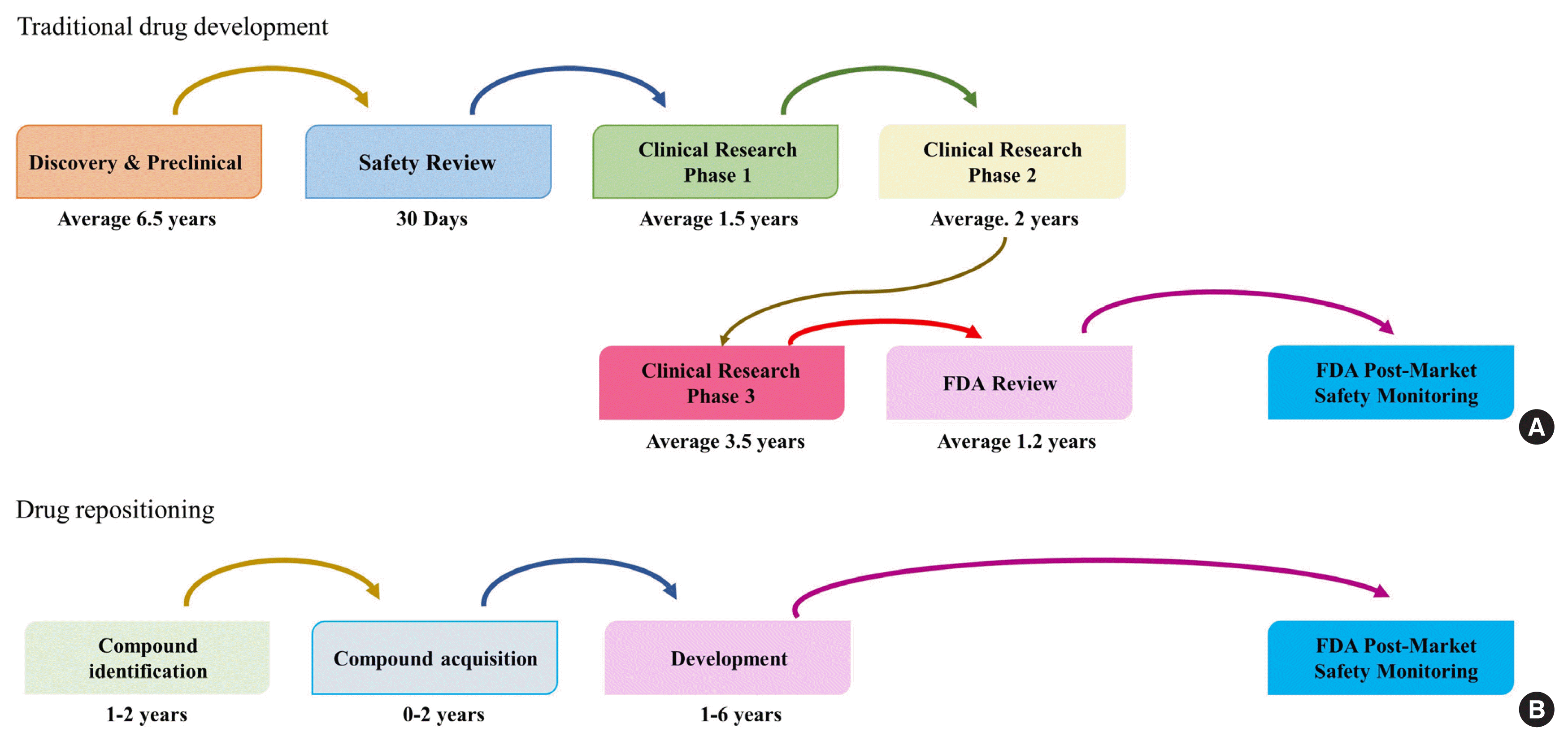
Traditional new drug development goes through the stages of discovery and preclinical trial, safety review, clinical research phases 1, 2, and 3, FDA review, and FDA post-market safety monitoring until a new drug is finally released in the market (Fig. 1) [7]. This process takes 12 to 16 years and costs an average of $12 billion. In contrast, drug repositioning is an efficient strategy that goes through the four steps of compound identification, acquisition, development, and post-market safety monitoring by the FDA. It is an effective strategy that takes half the time (average 6 years) when compared with the traditional new drug development period and costs only $1.6 billion [8–10]. Thus, it is possible to reduce the risk of new drug development failure by using a drug that has already been tested. The success rate of drug repositioning is typically less than 10%, which is relatively high [12].
In this issue of Endocrinology and Metabolism, Park et al. [13] published a study investigating the repositioning of candidate drugs for multiple diseases simultaneously. Although various studies have already addressed drug repositioning, the characteristic of this study is that it dealt with the National Health Insurance Service-National Sample Cohort (NHIS-NSC) data. The study design was detailed and systematic, and external validation using electronic medical records (EMR) was conducted based on evidence. This data-based approach, rather than the clinical approach, is distinctive. Although it is intended to reduce the risk of diabetic complications, the methodology in this process may be applicable to other drugs or other diseases. Particularly, the methodology using NHIS-NSC data, which researchers can easily access, may provide new insights to researchers interested in drug repositioning. As the interest in drug repositioning is expected to increase in the future, this study would be a good example. However, as the authors pointed out, the biggest limitation is that it is a retrospective study [14]. It was difficult to establish a causal relationship, and it was not possible to identify the existing confounding factors. Another limitation is that a specific disease cannot be clearly distinguished by an operational definition alone [15].
Despite these limitations, drug repositioning is considered an economical and efficient method as compared to the traditional new drug development method that will inevitably receive attention in the future. Recently, along with the issue of medical big data, the emergence of large-scale real world data, such as the EMR, NHIS, Health Insurance Review and Assessment Service, and Korea National Health and Nutrition Examination Survey, has provided new opportunities for many researchers interested in drug repositioning [16–20]. Efforts to find new indications for already approved drugs or new treatment methods for diseases will continue to increase in the future.
REFERENCES
1. Becker RC. Covid-19 treatment update: follow the scientific evidence. J Thromb Thrombolysis. 2020; 50:43–53.

2. Jarada TN, Rokne JG, Alhajj R. A review of computational drug repositioning: strategies, approaches, opportunities, challenges, and directions. J Cheminform. 2020; 12:46.

3. Yeu Y, Yoon Y, Park S. Protein localization vector propagation: a method for improving the accuracy of drug repositioning. Mol Biosyst. 2015; 11:2096–102.

4. Munos B. Lessons from 60 years of pharmaceutical innovation. Nat Rev Drug Discov. 2009; 8:959–68.

5. Saberian N, Peyvandipour A, Donato M, Ansari S, Draghici S. A new computational drug repurposing method using established disease-drug pair knowledge. Bioinformatics. 2019; 35:3672–8.

6. Low ZY, Farouk IA, Lal SK. Drug repositioning: new approaches and future prospects for life-debilitating diseases and the COVID-19 pandemic outbreak. Viruses. 2020; 12:1058.

7. Xue H, Li J, Xie H, Wang Y. Review of drug repositioning approaches and resources. Int J Biol Sci. 2018; 14:1232–44.

8. U.S. Food and Drug Administration. Drug development & approval process [Internet]. Silver Spring: FDA;2019. [cited 2022 Feb 3]. Available from: https://www.fda.gov/drugs/development-approval-process-drugs
.
10. Scannell JW, Blanckley A, Boldon H, Warrington B. Diagnosing the decline in pharmaceutical R&D efficiency. Nat Rev Drug Discov. 2012; 11:191–200.

11. Nuffer WA, Trujillo JM. Liraglutide: a new option for the treatment of obesity. Pharmacotherapy. 2015; 35:926–34.

12. Lotfi Shahreza M, Ghadiri N, Mousavi SR, Varshosaz J, Green JR. A review of network-based approaches to drug repositioning. Brief Bioinform. 2018; 19:878–92.

13. Park N, Jeon JY, Jeong E, Kim S, Yoon D. Drug repositioning using temporal trajectories of accompanying comorbidities in diabetes mellitus. Endocrinol Metab (Seoul). 2022; 37:65–73.

14. Kim HS, Kim JH. Proceed with caution when using real world data and real world evidence. J Korean Med Sci. 2019; 34:e28.

15. Kim HS, Kim DJ, Yoon KH. Medical big data is not yet available: why we need realism rather than exaggeration. Endocrinol Metab (Seoul). 2019; 34:349–54.

16. Lee S, Kim HS. Prospect of artificial intelligence based on electronic medical record. J Lipid Atheroscler. 2021; 10:282–90.

17. Kim L, Kim JA, Kim S. A guide for the utilization of Health Insurance Review and Assessment Service National Patient Samples. Epidemiol Health. 2014; 36:e2014008.

18. Kweon S, Kim Y, Jang MJ, Kim Y, Kim K, Choi S, et al. Data resource profile: the Korea National Health and Nutrition Examination Survey (KNHANES). Int J Epidemiol. 2014; 43:69–77.

19. Kyoung DS, Kim HS. Understanding and utilizing claim data from the Korean National Health Insurance Service (NHIS) and Health Insurance Review & Assessment (HIRA) database for research. J Lipid Atheroscler. 2021. Nov. 26. [Epub]. https://e-jla.org/DOIx.php?id=10.12997/jla.2022.11.e1
.

Fig. 1
Comparison between (A) traditional drug development and (B) drug repositioning. Modified from Xue et al. [7]. FDA, U.S. Food and Drug Administration.




 Citation
Citation Print
Print



 XML Download
XML Download