Abstract
Adrenal venous sampling (AVS) is the key procedure for lateralization of primary hyperaldosteronism (PA) before surgery. Identification of the adrenal veins using computed tomography (CT) and intraoperative cortisol assay facilitates the success of catheterization. Although administration of adrenocorticotropic hormone (ACTH) has benefits such as improving the success rate, some unilateral cases could be falsely diagnosed as bilateral. Selectivity index of 5 with ACTH stimulation to assess the selectivity of catheterization and lateralization index (LI) >4 with ACTH stimulation for unilateral diagnosis is used in many centers. Co-secretion of cortisol from the tumor potentially affects the lateralization by the LI. Patients aged <35 years with hypokalemia, marked aldosterone excess, and unilateral adrenal nodule on CT have a higher probability of unilateral disease. Patients with normokalemia, mild aldosterone excess, and no adrenal tumor on CT have a higher probability of bilateral disease. Although no methods have 100% specificity for subtype diagnosis that would allow bypassing AVS, prediction of the subtype should be considered when recommending AVS to patients. Methodological standardization and strict indication improve diagnostic quality of AVS. Development of non-invasive imaging and biochemical markers will drive a paradigm shift in the clinical practice of PA.
Primary hyperaldosteronism (PA) is one of the representative causes of secondary hypertension [1]. Subtype diagnosis, especially diagnosis of the unilateral subtype, which is treated by adrenal surgery, is the final and an important step of PA diagnosis. Adrenal venous sampling (AVS) has been used for over 50 years as the standard method to classify the PA subtype [2]. Since AVS is highly invasive and requires technical proficiency, adrenal computed tomography (CT) and adrenal scintigraphy replaced AVS as the first-choice method in the 1990s. However, the poor specificity of CT and poor sensitivity of scintigraphy, compared with the excellent specificity and sensitivity of AVS for detecting aldosterone-producing adenomas (APAs), have led to the revival of AVS as the gold standard for PA subtype classification and its recommendation by clinical practice guidelines [3,4] for over 20 years. Along with the awareness of PA as a common disease [5] and increased implementation of AVS in clinical practice, various approaches for technical improvement and standardization of AVS have been conducted. In this review, the methodological and clinical issues of AVS are reviewed.
To improve the success rate of AVS, it is important to standardize the protocol at each institution depending on the experience and available facilities [6]. Identification of adrenal vein anatomy by multi-detector row CT prior to AVS is essential [7]. It is particularly important to confirm the anatomy of the right adrenal vein, for which AVS has a lower success rate compared with the left adrenal vein. Although both sequential and simultaneous catheterization and blood sampling have been adopted, no report has shown any difference in the success rate between the two approaches. Whether to use the adrenocorticotropic hormone (ACTH) or not has been a matter of debate. Use of ACTH stimulation is generally recommended because of its benefits of excluding the effect of stress during the procedure and increasing the number of successful cannulations of both adrenal veins (see next section) [8].
Catheter insertion into the adrenal vein is confirmed by intraprocedural angiography, with special care not to damage the adrenal veins [9], and interventional 3D imaging (DynaCT) during AVS [10,11]. Intraprocedural measurement of the cortisol level in the adrenal veins enables judgment of AVS success [12]. More recently, a point-of-care testing device for semiquantitative measurement of cortisol levels on site has been developed [10,13]. The combination of imaging and a biochemical approach helps improve the success of AVS.
For evaluation of the AVS results, fluctuation of the cortisol level should be considered. Cortisol is a stress hormone that is secreted in a pulsatile fashion with diurnal rhythm. Since AVS is commonly associated with physical/psychological stress, cortisol levels may be significantly increased. This can affect the selectivity index (SI) used to evaluate successful cannulation and the lateralization index (LI) used for lateralization diagnosis [14]. Therefore, it is recommended to perform AVS without ACTH stimulation in the morning after rest and sedation for at least 15 minutes prior to minimize the effects of stress [9]. To minimize the effects of stress, AVS with ACTH stimulation is utilized in more than half of referral centers worldwide [15]. ACTH stimulation overwhelms stress-induced fluctuations in cortisol and aldosterone secretion, maximizes the cortisol level gradient between the adrenal veins and inferior vena cava, maximizes aldosterone secretion from APAs overexpressing the ACTH receptor (melanocortin 2 receptor [MC2R]), and improves the success rate of bilaterally selective catheterization [8,16,17].
On the other hand, the effects of ACTH stimulation on the diagnosis of lateralization are controversial [18]. Some studies showed that ACTH stimulation led to correct lateralization diagnosis, whereas others showed opposite results [16,19,20]. The Endocrine Society guideline neither recommends nor opposes the use of ACTH [3]. We recently demonstrated that ACTH stimulation decreased the LI and, in 22% of PA patients, changed the lateralization to bilateral from unilateral diagnosed without ACTH stimulation [21]. The patients who showed a change in the subtype from unilateral to bilateral could be divided into two subgroups. The first comprised the patients (57%) who showed a poor surgical outcome compared with the patients with a unilateral diagnosis regardless of ACTH stimulation, suggesting that lateralization by AVS with ACTH stimulation is more predictive of surgical outcome compared with AVS without ACTH stimulation. The unilateral lateralization by AVS without ACTH stimulation was assumed to be a false positive result. In contrast, the second subgroup comprised the patients (43%) who showed clinical and biochemical benefits after surgery and were diagnosed with adrenal adenomas by pathological analysis. This was the case especially when the LI in the absence of ACTH stimulation was greater than 8.3. The difference between the two subgroups could be attributed to the expression level of MC2R in the tumor: APAs with decreased expression of MC2R show an attenuated response of aldosterone secretion to ACTH and a decreased LI [22].
The method of ACTH stimulation (dosage, time of blood sampling, etc.) should be determined in advance [17,20]. If it is given via bolus, the time between ACTH administration and blood collection should be 15 to 30 minutes, and additional infusion is needed if blood collection is not completed by 45 to 60 minutes after stimulation. The recommended dose of ACTH is 250 μg for bolus administration or 250 μg over 3 to 5 hours (50 to 83.3 μg/hr) for infusion. AVS with ACTH stimulation is recommended, considering its benefits of abolishing the interfering effects of stress, improving the rate of bilaterally selective catheterization, and identifying APAs with a good surgical outcome, particularly in low-volume center. Whether to conduct AVS with or without ACTH stimulation should be determined in individual patients after considering the cost benefit, burden on the patient, hospital facilities, and health care framework.
The SI, defined as the ratio of the plasma cortisol concentration in the adrenal veins to that in the inferior vena cava, is used to assess the selectivity of adrenal catheterization based on a step up of the cortisol level between the adrenal vein and inferior vena cava. A questionnaire study on AVS conducted in 24 centers worldwide revealed that approximately 60% of the centers used a SI cutoff of 2 without ACTH stimulation and of 3 to 5 with ACTH stimulation, although the cutoff values varied among the centers [15]. A recent meta-analysis demonstrated that a SI of 1.1 to 3.0 without ACTH stimulation and 3 to 5 with ACTH stimulation are commonly used cutoff values [8]. In addition, AVS with ACTH stimulation significantly increased the number of successful cannulations compared with that without ACTH stimulation [8]. Although the cannulation success rate without ACTH stimulation decreased when the SI was increased from 1.1 to 5.0, that with ACTH stimulation was unchanged when the SI increased above 2 [16]. In contrast, the success rate based on a SI ≥1.4 without ACTH stimulation was recently demonstrated to correspond to that based on a SI ≥5 with ACTH stimulation [23]. Without ACTH stimulation, accurate lateralization was attained using a SI cutoff value of 1.1, and no further improvement was attained at higher cutoff values [24]. With ACTH stimulation, however, a SI ≥5 was needed to achieve accurate lateralization [25]. Comprehensively, the SI values of 2 without ACTH stimulation and 5 with ACTH stimulation have shown robustness in many centers and are therefore recommended.
Autonomous cortisol secretion (ACS) is experienced in approximately 30% of patients with APAs [26,27], which could affect SI. Several steroid metabolites and metanephrines are potential alternative markers verifying the success of adrenal vein cannulation [28,29]. However, a recent report suggested that an increased plasma cortisol level does not affect the SI under ACTH stimulation in PA patients with subclinical Cushing’s syndrome [30]. Thus, the SI may be adopted even in patients with cortisol-co-secreting APAs.
Determining the laterality of excess aldosterone secretion is the goal of AVS [3]. Several criteria have been advocated (Table 1). The LI is defined as the aldosterone to cortisol concentration ratio (A/C) of the dominant side divided by that of the non-dominant side of the adrenal vein. A LI >4 with ACTH stimulation is the most widely accepted criterion for unilateral diagnosis. In the Primary Aldosteronism Surgical Outcome (PASO) study, which verified the prognosis of unilateral PA and proposed an international consensus, the LI >4 with ACTH stimulation was accepted as an indication for unilateral adrenalectomy [31]. The validity of the LI >4 with ACTH stimulation was verified extensively in adrenalectomized cases and demonstrated as an independent predictor of a biochemical cure and postoperative outcome at 6 months after unilateral adrenalectomy, even in older patients [32]. On the contrary, a LI <4 with ACTH stimulation [33] has been demonstrated to substantially overlap with essential hypertension [34].
The contralateral aldosterone suppression ratio (CLR), which is defined as the ratio of the aldosterone to cortisol concentrations in the non-dominant adrenal vein divided by that in the inferior vena cava or peripheral vein after ACTH stimulation, is also employed to define unilateral PA [3,33]. A CLR <1 with ACTH stimulation has been used to define suppression of contralateral aldosterone secretion [35,36]. However, no previous reports have demonstrated that the CLR is an independent predictor of unilateral PA. The CLR is recommended to be utilized in combination with the LI [37,38].
As ACS is expected to affect the lateralization diagnosis as well as the success of AVS by altering the cortisol levels in the adrenal veins, the 1 mg Dexamethasone suppression test should be conducted in patients with PA prior to AVS, especially in patients with an apparent adrenal tumor on CT. An increased cortisol level on the tumor side decreases the A/C, while a decreased cortisol level on the contralateral side increases the A/C, which may lead to a decreased LI and an incorrect lateralization diagnosis when using the LI. However, O’Toole et al. [30] reported that mild ACS had a limited impact on AVS parameters when using the A/C with ACTH stimulation. The ratio of the plasma aldosterone concentration (PAC) in the dominant to that in the non-dominant adrenal vein could provide an alternative marker to the LI, although no standard cutoff value has been established for this ratio. If correcting the ACS takes a higher priority than correcting the aldosterone excess, which can be managed by mineralocorticoid receptor antagonists, surgical removal of the adrenal tumor with ACS may be advocated regardless of the PA laterality. In addition, total adrenalectomy of the side of cortisol secretion and partial adrenalectomy of the side of aldosterone secretion could be considered in patients with bilateral adrenal adenomas, which secrete cortisol and aldosterone independently [39].
Comprehensively, a LI of 4 with ACTH stimulation is recommended as the standard cutoff for predicting unilateral disease. A LI >2 without ACTH stimulation [9] and a CLR <1 with ACTH stimulation can be utilized complementarily to enforce the accuracy of unilateral diagnosis. Caution should be taken in the case of possible cortisol co-secretion considering its effect on the lateralization diagnosis.
When AVS is unsuccessful and results from one side of the adrenal vein and inferior vena cava (or peripheral vein) are available, the adrenal vein/inferior vena cava (AV/IVC) index, which is calculated by dividing the aldosterone/cortisol ratio in the adrenal vein by that in the inferior vena cava, was reported to be useful for predicting laterality. Pasternak et al. [40] demonstrated that an AV/IVC index of ≤0.5 is predictive of contralateral disease, while that of ≥5.5 is predictive of ipsilateral unilateral disease. However, a multi-institutional study by Wang et al. [41] failed to validate the 100% positive predictive value of this index reported in that study. Strajina et al. [42] reported that an AV/IVC index ≤0.5 performed well in identifying patients with contralateral unilateral disease, but an index ≥5.5 was not reliable in diagnosing ipsilateral unilateral disease. When AVS of the left adrenal vein is successful, a combination of an AV/IVC index <0.5 and aldosterone/cortisol ratio <9 in the left adrenal vein was shown to predict a right unilateral lesion [43]. The specificity and positive predictive value of the AV/IVC index, however, requires further validation before it can be used in place of other criteria.
No investigation has been conducted to determine a replacement for AVS as the gold standard for subtype diagnosis. However, considering various issues of AVS including invasiveness, technical requirement, cost, radiation exposure, and limited availability, it is reasonable to consider alternatives to skip AVS if applicable (Fig. 1). Patients with very high probability of unilateral disease for adrenal surgery and those with very high probability of bilateral disease for medical treatment are the candidates. Patients aged <35 years with spontaneous hypokalemia, marked aldosterone excess, and unilateral adrenal lesions on adrenal CT scan may not need AVS before unilateral adrenalectomy [3].
A recent study using the PA registry in Japan demonstrated that 90% of patients aged <35 years with hypokalemia, an elevated aldosterone level, and unilateral disease on CT showed concordance in the diagnosis by CT versus AVS. The concordance rate was lower in older patients [44]. In addition, the laterality defined by AVS was 100% concordant with the combination of unilateral disease on adrenal CT and factors including hypokalemia and a high estimated glomerular filtration rate in patients aged <40 years. CT findings alone could not confirm unilateral disease even in young patients [45].
In contrast, patients with a low PAC and aldosterone to renin ratio (ARR), obesity, female sex, normokalemia, and no adrenal tumors on CT [46–50] showed a higher risk of bilateral disease. Approximately 95% of patients with PA with normokalemia and normal adrenal CT findings had bilateral disease [46]. The prevalence of obesity was significantly higher in patients with bilateral PA compared with unilateral PA or essential hypertension [47]. Obesity and female sex were associated with bilateral PA, particularly in younger males and older females, respectively [48]. Clinical scoring of the subtype based on adrenal nodule absence on CT, low PAC and ARR, normokalemia, and female sex was predictive of bilateral PA in the validation cohort of the JPAS [49].
Nuclear imaging is another option for determining localization. Although scintigraphy using 131I-6beta-iodomethyl-19-norcholesterol as a tracer has been advocated traditionally, this probe is not specific for aldosterone production and requires dexamethasone suppression. In addition, its accumulation is affected by tumor size rather than functional activity [51], a limitation of its sensitivity. More recently, however, Yen et al. [52] reported that 131I-6beta-iodomethyl-19-norcholesterol single photon emission computed tomography (SPECT)/CT with dexamethasone suppression was useful for detecting APAs in patients with inconclusive AVS results.
Metomidate, the methylated version of etomidate, which is an imidazole-based potent inhibitor of steroid 11β-hydroxylase, is another candidate probe. Although 11C-metomidate positron emission tomography (PET), targeting CYP11B, has been reported to be useful for PA subtype classification [53], clinical application of this method is problematic due to the short half-life of 11C and the need for pretreatment with dexamethasone to block binding of the isotope to cortisol-producing CYP11B. In addition, Soinio et al. [54] reported that the concordance between AVS and 11C-metomidate PET was 55% in unilateral PA and 44% in bilateral PA and did not outperform adrenal CT. It was recently reported that the expression of chemokine receptor 4 (CXCR4), a receptor for inflammatory cytokines, is increased in aldosterone-producing tissue (particularly APA tissues) and is well correlated with the expression of CYP11B2. It was also reported that 68Ga-pentixafor PET/CT, targeting CXCR4 expression, is useful for determining the classification and lateralization of PA [55]. In addition, a CYP11B2-specific imaging agent has been developed [56,57]. Diagnosis by non-invasive imaging, which has excellent sensitivity, specificity, and cost effectiveness, is expected to provide an alternative to AVS.
New biomarkers for non-invasive subtype diagnosis have been identified. Steroid profiling by liquid chromatograph-mass spectrometry [58] and detection of increased 18-oxocortisol and 18-hydroxycortisol levels specifically in APAs harboring potassium inwardly rectifying channel subfamily J member 5 (KCNJ5) mutations [59,60] have been proposed as alternative methods to AVS for classifying the PA subtype. In addition, several microRNAs with differential expression in APA tissues and circulating blood [61,62] have been demonstrated as potential biomarkers useful for subtype diagnosis. Further studies to prove the specificity, sensitivity, and cost effectiveness are needed.
An updated overview of AVS based on stricter indications and standardized methods and decision criteria for evaluation of the results is illustrated in Fig. 2. AVS has various limitations that could interfere with its versatility and standardization. What is required for further improvement of AVS for subtype diagnosis? First, it is necessary to standardize the protocol including the need for ACTH stimulation and the optimal criteria for interpretation of the AVS results based on analysis of the postoperative outcome of PA. Second, it is necessary to reduce the invasiveness of AVS and patient burden by decreasing the test duration, radiation exposure, and complications of AVS. Third, stricter implementation and consideration of surgical candidacy prior to AVS are important to increase its diagnostic efficiency and utility [63]. Sufficient informed consent, including the probability of the expected subtype leading to the treatment choice, and a comprehensive decision considering the benefits/costs/disadvantages of AVS are essential before recommending AVS. Finally, development of non-invasive diagnostic methods including non-invasive imaging and biomarkers is expected. Non-invasive methods with comparable or better diagnostic ability, accuracy, safety, test duration, spread ability, cost effectiveness, and long-term postoperative outcomes compared with those of AVS will replace AVS as the gold standard, resulting in a paradigm shift in the clinical practice of PA
ACKNOWLEDGMENTS
This study was supported by the JPAS and JRAS from the Japan Agency for Medical Research and Development (AMED) under grant numbers JP17ek0109122 and JP20ek0109352 and by a research grant from the National Center for Global Health and Medicine, Japan (27-1402, 30-1008).
REFERENCES
1. Conn JW. Presidential address. I. Painting background. II. Primary aldosteronism, a new clinical syndrome. J Lab Clin Med. 1955; 45:3–17.
2. Melby JC, Spark RF, Dale SL, Egdahl RH, Kahn PC. Diagnosis and localization of aldosterone-producing adenomas by adrenal-vein cateterization. N Engl J Med. 1967; 277:1050–6.

3. Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment. An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016; 101:1889–916.

4. Funder JW, Carey RM, Fardella C, Gomez-Sanchez CE, Mantero F, Stowasser M, et al. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008; 93:3266–81.

5. Young WF. Primary aldosteronism: renaissance of a syndrome. Clin Endocrinol (Oxf). 2007; 66:607–18.

6. Young WF, Stanson AW. What are the keys to successful adrenal venous sampling (AVS) in patients with primary aldosteronism? Clin Endocrinol (Oxf). 2009; 70:14–7.

7. Ota H, Seiji K, Kawabata M, Satani N, Omata K, Ono Y, et al. Dynamic multidetector CT and non-contrast-enhanced MR for right adrenal vein imaging: comparison with catheter venography in adrenal venous sampling. Eur Radiol. 2016; 26:622–30.

8. Laurent I, Astere M, Zheng F, Chen X, Yang J, Cheng Q, et al. Adrenal venous sampling with or without adrenocorticotropic hormone stimulation: a meta-analysis. J Clin Endocrinol Metab. 2019; 104:1060–8.

9. Rossi GP, Auchus RJ, Brown M, Lenders JW, Naruse M, Plouin PF, et al. An expert consensus statement on use of adrenal vein sampling for the subtyping of primary aldosteronism. Hypertension. 2014; 63:151–60.

10. Chang CC, Lee BC, Chang YC, Wu VC, Huang KH, Liu KL, et al. Comparison of C-arm computed tomography and on-site quick cortisol assay for adrenal venous sampling: a retrospective study of 178 patients. Eur Radiol. 2017; 27:5006–14.

11. Park CH, Hong N, Han K, Kang SW, Lee CR, Park S, et al. C-Arm computed tomography-assisted adrenal venous sampling improved right adrenal vein cannulation and sampling quality in primary aldosteronism. Endocrinol Metab (Seoul). 2018; 33:236–44.

12. Stowasser M. Improving the success and reliability of adrenal venous sampling: focus on intraprocedural cortisol measurement. Clin Chem. 2012; 58:1275–7.

13. Yoneda T, Karashima S, Kometani M, Usukura M, Demura M, Sanada J, et al. Impact of new quick gold nanoparticle-based cortisol assay during adrenal vein sampling for primary aldosteronism. J Clin Endocrinol Metab. 2016; 101:2554–61.

14. Seccia TM, Miotto D, Battistel M, Motta R, Barisa M, Maniero C, et al. A stress reaction affects assessment of selectivity of adrenal venous sampling and of lateralization of aldosterone excess in primary aldosteronism. Eur J Endocrinol. 2012; 166:869–75.

15. Rossi GP, Barisa M, Allolio B, Auchus RJ, Amar L, Cohen D, et al. The Adrenal Vein Sampling International Study (AVIS) for identifying the major subtypes of primary aldosteronism. J Clin Endocrinol Metab. 2012; 97:1606–14.

16. Rossi GP, Pitter G, Bernante P, Motta R, Feltrin G, Miotto D. Adrenal vein sampling for primary aldosteronism: the assessment of selectivity and lateralization of aldosterone excess baseline and after adrenocorticotropic hormone (ACTH) stimulation. J Hypertens. 2008; 26:989–97.

17. Takeda Y, Umakoshi H, Takeda Y, Yoneda T, Kurihara I, Katabami T, et al. Impact of adrenocorticotropic hormone stimulation during adrenal venous sampling on outcomes of primary aldosteronism. J Hypertens. 2019; 37:1077–82.

18. Deinum J, Groenewoud H, van der Wilt GJ, Lenzini L, Rossi GP. Adrenal venous sampling: cosyntropin stimulation or not? Eur J Endocrinol. 2019; 181:D15–26.

19. Satoh F, Abe T, Tanemoto M, Nakamura M, Abe M, Uruno A, et al. Localization of aldosterone-producing adrenocortical adenomas: significance of adrenal venous sampling. Hypertens Res. 2007; 30:1083–95.

20. Monticone S, Satoh F, Giacchetti G, Viola A, Morimoto R, Kudo M, et al. Effect of adrenocorticotropic hormone stimulation during adrenal vein sampling in primary aldosteronism. Hypertension. 2012; 59:840–6.

21. Kobayashi H, Nakamura Y, Abe M, Kurihara I, Itoh H, Ichijo T, et al. Effect of cosyntropin during adrenal venous sampling on subtype of primary aldosteronism: analysis of surgical outcome. Eur J Endocrinol. 2020; 182:265–73.

22. Rossitto G, Maiolino G, Lenzini L, Bisogni V, Seccia TM, Cesari M, et al. Subtyping of primary aldosteronism with adrenal vein sampling: hormone- and side-specific effects of cosyntropin and metoclopramide. Surgery. 2018; 163:789–95.

23. Rossitto G, Amar L, Azizi M, Riester A, Reincke M, Degenhart C, et al. Subtyping of primary aldosteronism in the AVIS-2 Study: assessment of selectivity and lateralization. J Clin Endocrinol Metab. 2020; 105:dgz017.

24. Rossi GP, Sacchetto A, Chiesura-Corona M, De Toni R, Gallina M, Feltrin GP, et al. Identification of the etiology of primary aldosteronism with adrenal vein sampling in patients with equivocal computed tomography and magnetic resonance findings: results in 104 consecutive cases. J Clin Endocrinol Metab. 2001; 86:1083–90.

25. Ceral J, Solar M, Krajina A, Ballon M, Suba P, Cap J. Adrenal venous sampling in primary aldosteronism: a low dilution of adrenal venous blood is crucial for a correct interpretation of the results. Eur J Endocrinol. 2010; 162:101–7.

26. Akehi Y, Yanase T, Motonaga R, Umakoshi H, Tsuiki M, Takeda Y, et al. High prevalence of diabetes in patients with primary aldosteronism (PA) associated with subclinical hypercortisolism and prediabetes more prevalent in bilateral than unilateral PA: a large, multicenter cohort study in Japan. Diabetes Care. 2019; 42:938–45.

27. Gerards J, Heinrich DA, Adolf C, Meisinger C, Rathmann W, Sturm L, et al. Impaired glucose metabolism in primary aldosteronism is associated with cortisol cosecretion. J Clin Endocrinol Metab. 2019; 104:3192–202.

28. Lenders JWM, Eisenhofer G, Reincke M. Subtyping of patients with primary aldosteronism: an update. Horm Metab Res. 2017; 49:922–8.

29. Goupil R, Wolley M, Ahmed AH, Gordon RD, Stowasser M. Does concomitant autonomous adrenal cortisol overproduction have the potential to confound the interpretation of adrenal venous sampling in primary aldosteronism? Clin Endocrinol (Oxf). 2015; 83:456–61.

30. O’Toole SM, Sze WC, Chung TT, Akker SA, Druce MR, Waterhouse M, et al. Low-grade cortisol cosecretion has limited impact on ACTH-stimulated AVS parameters in primary aldosteronism. J Clin Endocrinol Metab. 2020; 105:dgaa519.

31. Williams TA, Lenders JWM, Mulatero P, Burrello J, Rottenkolber M, Adolf C, et al. Outcomes after adrenalectomy for unilateral primary aldosteronism: an international consensus on outcome measures and analysis of remission rates in an international cohort. Lancet Diabetes Endocrinol. 2017; 5:689–99.

32. Takeda M, Yamamoto K, Akasaka H, Rakugi H, Naruse M, Takeda Y, et al. Clinical characteristics and postoperative outcomes of primary aldosteronism in the elderly. J Clin Endocrinol Metab. 2018; 103:3620–9.

33. Nishikawa T, Omura M, Satoh F, Shibata H, Takahashi K, Tamura N, et al. Guidelines for the diagnosis and treatment of primary aldosteronism: the Japan Endocrine Society 2009. Endocr J. 2011; 58:711–21.

34. Umakoshi H, Naruse M, Wada N, Ichijo T, Kamemura K, Matsuda Y, et al. Adrenal venous sampling in patients with positive screening but negative confirmatory testing for primary aldosteronism. Hypertension. 2016; 67:1014–9.

35. Kline GA, Chin A, So B, Harvey A, Pasieka JL. Defining contralateral adrenal suppression in primary aldosteronism: implications for diagnosis and outcome. Clin Endocrinol (Oxf). 2015; 83:20–7.

36. Wolley MJ, Gordon RD, Ahmed AH, Stowasser M. Does contralateral suppression at adrenal venous sampling predict outcome following unilateral adrenalectomy for primary aldosteronism? A retrospective study. J Clin Endocrinol Metab. 2015; 100:1477–84.

37. Umakoshi H, Tanase-Nakao K, Wada N, Ichijo T, Sone M, Inagaki N, et al. Importance of contralateral aldosterone suppression during adrenal vein sampling in the subtype evaluation of primary aldosteronism. Clin Endocrinol (Oxf). 2015; 83:462–7.

38. Lee J, Kang B, Ha J, Kim MH, Choi B, Hong TH, et al. Clinical outcomes of primary aldosteronism based on lateralization index and contralateral suppression index after adrenal venous sampling in real-world practice: a retrospective cohort study. BMC Endocr Disord. 2020; 20:114.

39. Lee SE, Kim JH, Lee YB, Seok H, Shin IS, Eun YH, et al. Bilateral adrenocortical masses producing aldosterone and cortisol independently. Endocrinol Metab (Seoul). 2015; 30:607–13.

40. Pasternak JD, Epelboym I, Seiser N, Wingo M, Herman M, Cowan V, et al. Diagnostic utility of data from adrenal venous sampling for primary aldosteronism despite failed cannulation of the right adrenal vein. Surgery. 2016; 159:267–73.

41. Wang TS, Kline G, Yen TW, Yin Z, Liu Y, Rilling W, et al. A multi-institutional comparison of adrenal venous sampling in patients with primary aldosteronism: caution advised if successful bilateral adrenal vein sampling is not achieved. World J Surg. 2018; 42:466–72.

42. Strajina V, Al-Hilli Z, Andrews JC, Bancos I, Thompson GB, Farley DR, et al. Primary aldosteronism: making sense of partial data sets from failed adrenal venous sampling-suppression of adrenal aldosterone production can be used in clinical decision making. Surgery. 2018; 163:801–6.

43. Fujii Y, Umakoshi H, Wada N, Ichijo T, Kamemura K, Matsuda Y, et al. Subtype prediction of primary aldosteronism by combining aldosterone concentrations in the left adrenal vein and inferior vena cava: a multicenter collaborative study on adrenal venous sampling. J Hum Hypertens. 2017; 32:12–9.

44. Umakoshi H, Ogasawara T, Takeda Y, Kurihara I, Itoh H, Katabami T, et al. Accuracy of adrenal computed tomography in predicting the unilateral subtype in young patients with hypokalaemia and elevation of aldosterone in primary aldosteronism. Clin Endocrinol (Oxf). 2018; 88:645–51.

45. Riester A, Fischer E, Degenhart C, Reiser MF, Bidlingmaier M, Beuschlein F, et al. Age below 40 or a recently proposed clinical prediction score cannot bypass adrenal venous sampling in primary aldosteronism. J Clin Endocrinol Metab. 2014; 99:E1035–9.

46. Umakoshi H, Tsuiki M, Takeda Y, Kurihara I, Itoh H, Katabami T, et al. Significance of computed tomography and serum potassium in predicting subtype diagnosis of primary aldosteronism. J Clin Endocrinol Metab. 2018; 103:900–8.

47. Ohno Y, Sone M, Inagaki N, Yamasaki T, Ogawa O, Takeda Y, et al. Obesity as a key factor underlying idiopathic hyperaldosteronism. J Clin Endocrinol Metab. 2018; 103:4456–64.

48. Akasaka H, Yamamoto K, Rakugi H, Nagasawa M, Nakamaru R, Ichijo T, et al. Sex difference in the association between subtype distribution and age at diagnosis in patients with primary aldosteronism. Hypertension. 2019; 74:368–74.

49. Kobayashi H, Abe M, Soma M, Takeda Y, Kurihara I, Itoh H, et al. Development and validation of subtype prediction scores for the workup of primary aldosteronism. J Hypertens. 2018; 36:2269–76.

50. Xiao L, Jiang Y, Zhang C, Jiang L, Zhou W, Su T, et al. A novel clinical nomogram to predict bilateral hyperaldosteronism in Chinese patients with primary aldosteronism. Clin Endocrinol (Oxf). 2019; 90:781–8.

51. Nomura K, Kusakabe K, Maki M, Ito Y, Aiba M, Demura H. Iodomethylnorcholesterol uptake in an aldosteronoma shown by dexamethasone-suppression scintigraphy: relationship to adenoma size and functional activity. J Clin Endocrinol Metab. 1990; 71:825–30.

52. Yen RF, Wu VC, Liu KL, Cheng MF, Wu YW, Chueh SC, et al. 131I-6beta-iodomethyl-19-norcholesterol SPECT/CT for primary aldosteronism patients with inconclusive adrenal venous sampling and CT results. J Nucl Med. 2009; 50:1631–7.

53. Burton TJ, Mackenzie IS, Balan K, Koo B, Bird N, Soloviev DV, et al. Evaluation of the sensitivity and specificity of (11)C-metomidate positron emission tomography (PET)-CT for lateralizing aldosterone secretion by Conn’s adenomas. J Clin Endocrinol Metab. 2012; 97:100–9.
54. Soinio M, Luukkonen AK, Seppanen M, Kemppainen J, Seppanen J, Pienimaki JP, et al. Functional imaging with 11C-metomidate PET for subtype diagnosis in primary aldosteronism. Eur J Endocrinol. 2020; 183:539–50.

55. Ding J, Zhang Y, Wen J, Zhang H, Wang H, Luo Y, et al. Imaging CXCR4 expression in patients with suspected primary hyperaldosteronism. Eur J Nucl Med Mol Imaging. 2020; 47:2656–65.

56. Abe T, Naruse M, Young WF Jr, Kobashi N, Doi Y, Izawa A, et al. A novel CYP11B2-specific imaging agent for detection of unilateral subtypes of primary aldosteronism. J Clin Endocrinol Metab. 2016; 101:1008–15.

57. Sander K, Gendron T, Cybulska KA, Sirindil F, Zhou J, Kalber TL, et al. Development of [18F]AldoView as the first highly selective aldosterone synthase PET tracer for imaging of primary hyperaldosteronism. J Med Chem. 2021; 64:9321–29.
58. Eisenhofer G, Duran C, Cannistraci CV, Peitzsch M, Williams TA, Riester A, et al. Use of steroid profiling combined with machine learning for identification and subtype classification in primary aldosteronism. JAMA Netw Open. 2020; 3:e2016209.

59. Satoh F, Morimoto R, Ono Y, Iwakura Y, Omata K, Kudo M, et al. Measurement of peripheral plasma 18-oxocortisol can discriminate unilateral adenoma from bilateral diseases in patients with primary aldosteronism. Hypertension. 2015; 65:1096–102.

60. Tezuka Y, Yamazaki Y, Kitada M, Morimoto R, Kudo M, Seiji K, et al. 18-Oxocortisol synthesis in aldosterone-producing adrenocortical adenoma and significance of KCNJ5 mutation status. Hypertension. 2019; 73:1283–90.

61. Nakano Y, Yoshimoto T, Watanabe R, Murakami M, Fukuda T, Saito K, et al. miRNA299 involvement in CYP11B2 expression in aldosterone-producing adenoma. Eur J Endocrinol. 2019; 181:69–78.

Fig. 1
Clinical findings to bypass adrenal venous sampling (AVS) and select the appropriate treatment. As no method has 100% specificity for subtype diagnosis, AVS is indicated if the patient agrees to undergo AVS. PA, primary aldosteronism; BMI, body mass index; CT, computed tomography.
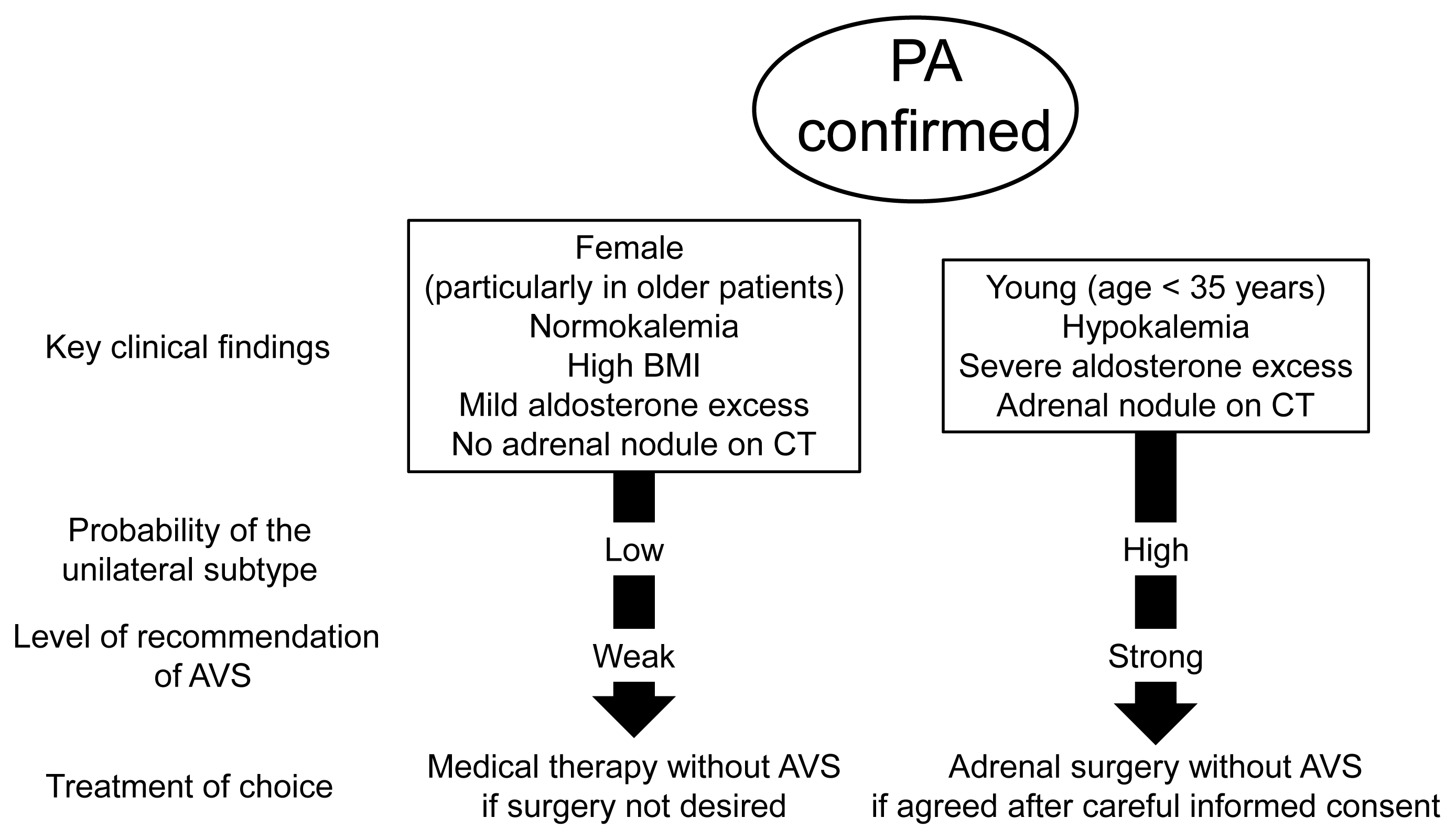
Fig. 2
Strategic and organized implementation of adrenal venous sampling (AVS) based on stricter indications and standardized methods and decision criteria for evaluation of the results. PA, primary aldosteronism; MDCT, multi-detector row computed tomography; ACTH, adrenocorticotropic hormone; SI, selectivity index; LI, lateralization index; CR, contralateral suppression.
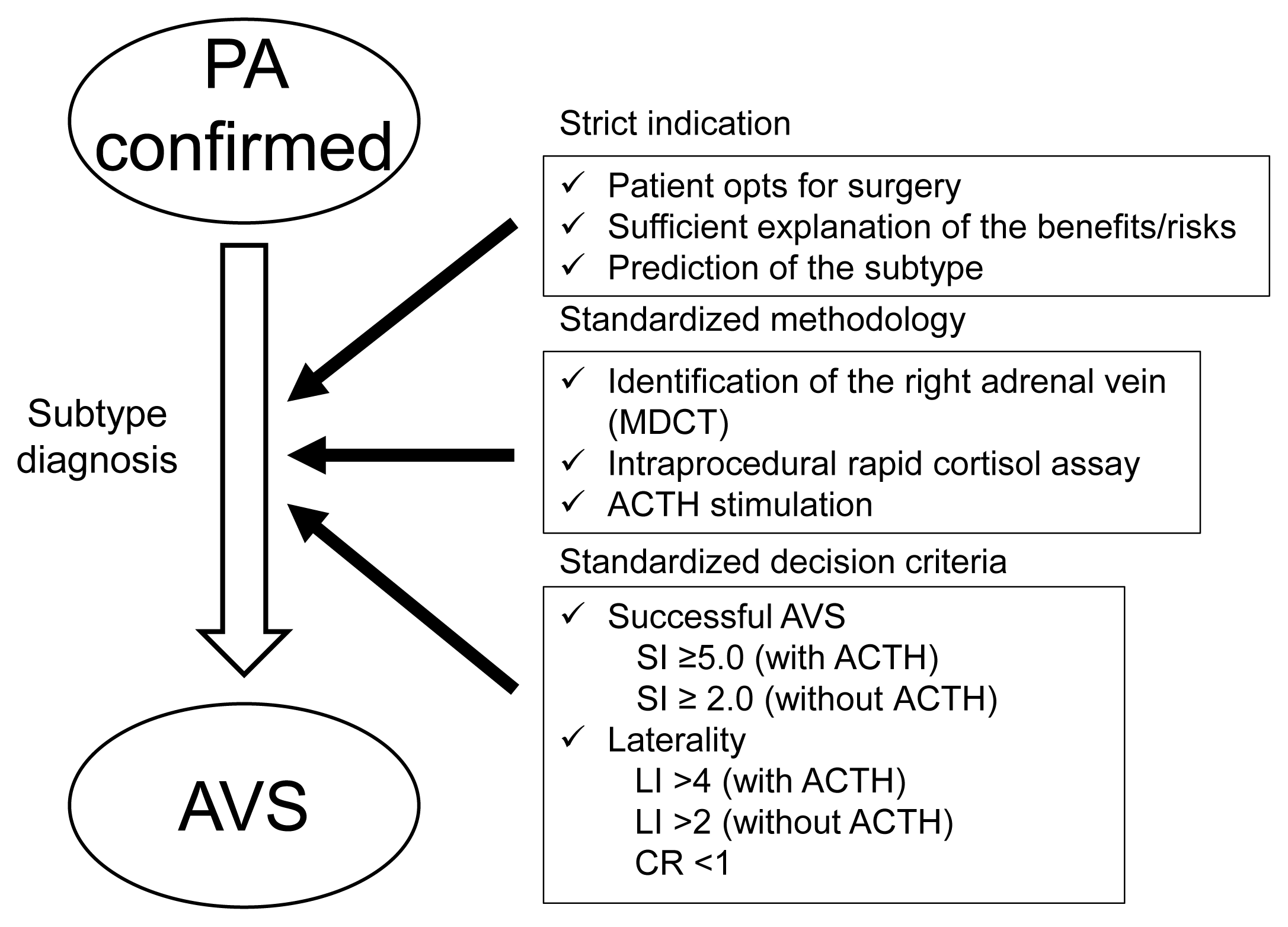
Table 1
Definition and Clinical Utility of the Lateralization Criteria
| Criteria | Definition | Cutoff value | Clinical significance |
|---|---|---|---|
| Lateralization index (LI) | (PACDOM/PCCDOM)/(PACNONDOM/PCCNONDOM) | >4 (>2a) | Aldosterone secretion is significantly increased from dominant side compared with non-dominant side [3,9,14,30]. |
| Contralateral aldosterone suppression ratio (CR) | (PACNONDOM/PCCNONDOM)/(PACIVC or PV/PCCIVC or PV) | <1 | Aldosterone secretion is significantly suppressed from non-dominant side compared with IVC or PV [3,30,32,35]. |
| AV/IVC ratio | (PACAV/PCCAV)/(PACIVC or PV/PCCIVC or PV) |
>5.5 (ipsilateral unilateral) <0.5 (contralateral unilateral) |
Aldosterone secretion from ipsilateral side is significantly increased or that from contralateral side is suppressed [40,42]. |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download