Abstract
Intermittent fasting has become an increasingly popular strategy in losing weight and associated reduction in obesity-related medical complications. Overwhelming studies support metabolic improvements from intermittent fasting in blood glucose levels, cardiac and brain function, and other health benefits, in addition to weight loss. However, concerns have also been raised on side effects including muscle loss, ketosis, and electrolyte imbalance. Of particular concern, the effect of intermittent fasting on hormonal circadian rhythms has received little attention. Given the known importance of circadian hormonal changes to normal physiology, potential detrimental effects by dysregulation of hormonal changes deserve careful discussions. In this review, we describe the changes in circadian rhythms of hormones caused by intermittent fasting. We covered major hormones commonly pathophysiologically involved in clinical endocrinology, including insulin, thyroid hormones, and glucocorticoids. Given that intermittent fasting could alter both the level and frequency of hormone secretion, decisions on practicing intermittent fasting should take more considerations on potential detrimental consequences versus beneficial effects pertaining to individual health conditions.
Technological breakthroughs have caused dramatic changes in human lifestyle. One typical example is that people can work and eat in the evening and even at night, contrary to the traditional diurnal lifestyle of humans [1]. Cumulative evidence suggests that these lifestyle changes increase the risk of metabolic disorders such as obesity and type 2 diabetes [2].
Intermittent fasting has gained popularity to manage obesity and diabetes. It refers to various diet regimens that involve periodically conducted fasting [3]. This protocol is relatively easy to practice for long periods of time compared to continuous calorie reduction (CR), which requires inconvenient calorie counting every day [4]. One simple approach is alternate-day fasting (ADF), which alternates a day of complete or considerable food restriction with ad libitum food intake for another day. Whole-day fasting (periodic fasting) involves complete or considerable food restriction for 1 to 2 days in a week: for example, the 5:2 diet is to restrict calories by around 75% for 2 days a week. Time-restricted feeding (TRF) allows subjects to eat only for a specific time window during the day. Ramadan fasting, a kind of religious ritual, can be categorized as a type of TRF since meals are consumed only between sunset and dawn during this period.
Beneficial effects of intermittent fasting have been extensively reviewed in recent papers [5–8]. For example, intermittent fasting can improve cognitive and learning abilities in rodents [9,10] and activate antioxidant enzymes and autophagy to resist cellular stress [11,12]. In humans, several studies demonstrate that intermittent fasting decreases body weight, plasma glucose/lipid levels, blood pressure, and inflammation markers [3,13]. On the contrary, other clinical studies showed that intermittent fasting had little effect on weight loss [14,15]. Moreover, it may have potential side effects such as electrolyte imbalance and muscle loss [16,17].
Living animals have biological clocks, called circadian rhythms, which control many aspects of physiological function on a 24-hour cycle. This includes the sleep-wake cycle, blood pressure, heart beat, body temperature and hormonal production/secretion [18,19]. The master clock of systemic circadian rhythms is located in the suprachiasmatic nucleus (SCN) of the hypothalamus, whereas peripheral clocks are distributed in a number of cells throughout the body. The SCN is remarkable in that it is both autonomous and entrained by external time cues. The intra-SCN cellular network endows exceptional robustness to the molecular and neural oscillation of the SCN cellular population, allowing the self-sustainability required to be the master clock [20]. The SCN is also directly innervated by intrinsically photosensitive retinal ganglion cells, enabling it to adjust the circadian phase by utilizing light as a time cue. It then transmits circadian information to peripheral organs through (1) its projection to downstream brain regions, including the paraventricular nucleus of the hypothalamus, dorsomedial hypothalamus, and arcuate nucleus and (2) humoral cues, such as glucocorticoids through its regulation of the hypothalamus-pituitary-adrenal gland (HPA) axis [21].
The autoregulatory feedback loops of transcription–translation serve as the molecular basis of cellular clockworks [20]. Key clockwork transcription factors circadian locomotor output cycles kaput (CLOCK) and brain and muscle Arnt-like protein 1 (BMAL1) form a heterodimeric complex and bind to the promoter of clock genes (period 1–3 and cryptochrome 1/2) to stimulate their production. The protein products of these genes suppress CLOCK/BMAL1, preventing their own transcription [22,23]. Such positive and negative feedback loops generate the cyclic oscillation of clock gene expression over a 24-hour period. This mechanism is found in almost all types of cells, forming the “peripheral clocks” which are regulated by the master clock [21]. Neuroendocrine and endocrine cells are no exception and reportedly harbor functional clockwork, linking the circadian system to hormonal synthesis and secretion.
Recent review papers [3,13,24,25] on intermittent fasting have mainly dealt with changes in anthropometric indices (e.g., body weight, waist circumference, total fat mass), cardiovascular risk factors (e.g., blood pressure, heart beat), metabolite levels (e.g., glucose, cholesterol, triglyceride, ketone bodies), and levels of glucoregulatory hormones (e.g., insulin, leptin, adiponectin). To our knowledge, reviews summarizing changes in hormonal rhythms caused by intermittent fasting are scarce. Therefore, in this review, we will summarize reports on the hormonal alterations caused by intermittent fasting in mammals, especially focusing on their circadian pattern.
As humans and rodents have opposite circadian patterns, research data obtained from human and animal studies must be interpreted with caution. Laboratory rodents are nocturnal and normally fed ad libitum; they consume 90% of foods during the dark cycle and mostly sleep during the light period. In contrast, humans, especially in the period of hunter-gatherers, usually work and eat during the daytime.
In the following paragraphs, we have summarized hormonal changes that have been observed during or after intermittent fasting: the shift in acrophase (the time when hormonal levels reach maximum) and alterations in the amplitude and frequency of daily endocrine rhythms. Moreover, we have described the acute consequences of fasting on various hormones, as studies regarding the effect of intermittent fasting on such hormones are lacking.
Insulin is a major metabolic hormone produced in and secreted from pancreatic β cells [26]. In target tissues such as skeletal muscles and adipose tissues, insulin stimulates glucose uptake and storage of excessive energy as glycogen and lipids. Insulin also inhibits glucose production in the liver, thereby lowering postprandial blood sugar to maintain normal blood glucose levels [27].
Interestingly, evidence suggests that insulin production and release in pancreatic β cells is partly regulated by clock proteins. Genetic ablation of clock genes in pancreatic β cells abolished the circadian pattern of insulin secretion and decreased baseline insulin levels below the nadir value of wildtype mice [28,29]. CLOCK and BMAL1 proteins bind to regulatory elements and regulate the expression of genes involved in insulinotropic signals and insulin exocytosis [28,29]. The availability of substrates also plays a part in regulating insulin secretion. Upon rises in blood glucose levels following meal ingestion, pancreatic β cells take in and oxidize glucose to produce adenosine triphosphate (ATP). Elevated ATP closes ATP-dependent inhibitory K+ channels, depolarizing the plasma membrane. Subsequently, voltage-dependent Ca2+ channels open and extracellular Ca2+ enters into the β cells, leading to Ca2+-mediated exocytosis of insulin secretory vesicles [30–32]. Consequently, meal-dependent fluctuation of insulin shows an instant postprandial rise and a subsequent decline until the next meal (Figs. 1A, 2A) [33,34]. In laboratory mice, baseline (between meals) plasma insulin levels followed a circadian rhythm with a nocturnal (light-off) peak, which correlated with the diurnal pattern of plasma leptin [35]. In addition to insulin production and secretion, insulin sensitivity also exhibits distinct circadian rhythmicity and partly depends on the local molecular clocks of insulin-sensitive organs [36]. For example, skeletal muscle-specific Bmal1 knockout mice showed impaired insulin-stimulated glucose uptake in skeletal muscle [37]. This study proposed that BMAL1 depletion was associated with decreased levels of glucose transporter type 4 (GLUT4), the insulin-responsive glucose transporter, and the resultant decrease in insulin sensitivity. Another animal experiment revealed that clock proteins, including Rev-Erbα, negatively regulate the expression of the retinol receptor stimulated by retinoic acid 6 (STRA6) in white adipose tissue. STRA6 activation results in decreased insulin sensitivity under normal physiological conditions; therefore, the diurnal variation of STRA6 is one of the molecular mechanisms that mediate the circadian rhythm of insulin sensitivity [38].
Starvation for 24 hours in mice decreased plasma insulin levels dramatically and abolished the normal circadian rhythm compared with ad libitum mice [35]. Similarly, two ADF studies (3 months and 20 weeks, respectively) in mice reported a dramatic reduction in serum insulin levels by the last week of diet but gave different explanations about this phenotype. In one study [39], total food intake of ADF group mice was less than that of ad libitum group mice, and thus the reduced energy intake was thought to have caused decreased insulin secretion. In the other study [40], total food intake of the ADF group was similar to ad libitum mice because ADF mice consumed twice as much food on fed-days. Therefore, ADF may reduce insulin levels independent of total energy intake, as opposed to CR. On the other hand, the effect of TRF was different from ADF, possibly due to food anticipation. For example, 3 week-TRF in rats (feeding for 8 hours with variable fasting periods) showed a shift in the acrophase (from 9:00 PM to 12:00–1:00 PM) and doubled peak insulin levels compared to ad libitum rats [41].
Likewise, in humans, intermittent fasting decreased plasma insulin levels and improved insulin sensitivity. This change has already been observed during acute fasting. For example, upon 72 hour-fasting, plasma insulin dropped by about 35% during the first 24 hours and reached half of baseline levels by the end of fasting (Fig. 1A) [42]. ADF for 22 days dramatically suppressed insulin secretion by 50% [43]. In studies involving 5:2 diets for 3 or 6 months, blood insulin levels and insulin resistance were significantly reduced. These changes were greater than those induced by CR although the two diets induced comparable weight loss [7,44]. A recent study on 8-week TRF reported similar results [45]. In these human studies, circadian rhythms of insulin levels were not reported. As the consistent reduction in insulin levels induced by intermittent fasting in humans seems to be related to, but not fully explained by, energy restriction, further investigation of its mechanisms, such as changes in pancreatic β cell function or insulin clearance rates, will be needed in the future.
Thyroid hormones (triiodothyronine [T3] and thyroxine [T4]) are iodine-containing hormones released from the thyroid gland [46]. Thyroid hormones promote thermogenesis in brown adipose tissue by increasing uncoupling protein expression. In white adipose tissues, thyroid hormones accelerate lipolysis, increasing fatty acids that the liver uses for gluconeogenesis. Thyroid hormones stimulate ATPases in skeletal muscle, which promotes energy expenditure [47].
The hypothalamus-pituitary-thyroid axis (HPT axis) is one of the central pathways regulating energy expenditure: hypothalamic thyrotropin-releasing hormone (TRH) promotes the secretion of thyroid-stimulating hormone (TSH) from the anterior pituitary gland. TSH stimulates the thyroid to release T4, which is later converted to active T3 by type I and II deiodinases [48]. The HPT axis is controlled by the SCN through dual mechanisms: hormonal and neural. Tracing by pseudorabies virus in rats showed that SCN neurons project to TRH-expressing neurons in the hypothalamic paraventricular nucleus and directly innervate the thyroid gland [49]. In addition, local clocks in thyrotropes may contribute to the circadian expression of TSH via rhythmic transcriptional repression of REV-ERBα, as suggested by an in vitro study using TαT1.1, a thyrotrope cell line [50]. Under the control of circadian clocks, thyroid hormones peak in the early phase of inactive periods: in nocturnal rodents, TSH peaks at 10:30 AM–12:30 PM and T3 at 11:00 AM–3:00 PM [51]. Inversely, in humans, TSH level is highest at 2:00–4:00 AM, and T3 secretion subsequently peaks 1.5 hours after (at 3:30–5:30 AM). The trough of T3 levels is observed at 5:00–6:00 PM (Figs. 1B, 2B) [52,53].
In rodent experiments, complete fasting for 24 hours lowers serum T3. This might result from the increased activity of type III deiodinase, which inactivates thyroid hormones [54,55]. Contrary to primary hypothyroidism, in which both TRH and TSH are upregulated as a compensatory response, fasting decreases TRH and TSH levels [56]. This difference could be mediated by alterations of leptin and neuromedin B: restoring leptin concentrations during fasting ameliorated TRH decrease [57] because leptin stimulates hypothalamic TRH neurons via the melanocortin-dependent pathway [58,59]. Meanwhile, pituitary neuromedin B suppresses TSH release, and the proportion of neuromedin B in anterior pituitary proteins increased during fasting [60].
In humans, the T3 level starts to decrease rapidly after fasting. When measured during 80-hour fasting in healthy subjects, marked T3 and TSH reductions were observed within 48 hours from fasting onset (Fig. 1B) [61]. Another trial reported that serum T3 decreased up to 55% after 24 hours of fasting. Contrary to serum T3, TSH levels remained unchanged after fasting [62]. Short-term (4 weeks) and long-term (more than 6 months) ADF diets reduced circulating T3 without any change in TSH level [63]. The same result was obtained from another 8-hour TRF (isocaloric, ad libitum within the defined time) study for 8 weeks [64].
Glucocorticoids (corticosterone in rodents and cortisol in humans) are synthesized and secreted by the adrenal cortex. Glucocorticoids stimulate the breakdown of macromolecules and counter-regulate insulin to maintain glucose homeostasis. They also induce anti-inflammatory responses and cardiovascular hypertonicity [65].
The circadian control of blood glucocorticoid levels occurs through multiple mechanisms: hormonal cascade in the HPA cortex axis, autonomic neural signals, and local clocks residing in the adrenal cortex. Neural projections from the SCN to the hypothalamic paraventricular nucleus (PVH) activate the release of corticotropin-releasing hormone (CRH). This stimulates the pituitary to secrete adrenocorticotropic hormone (ACTH), which finally induces glucocorticoid secretion from the adrenal gland [66]. Recent studies have demonstrated a role for PVH neurons including CRH neurons in obesity development via circadian regulation [67,68]. Autonomic nerves from the SCN to the adrenal gland take another part in glucocorticoid release [69,70]. Besides, clock proteins, CLOCK and BMAL1, heterodimerize and bind directly to the promoter of steroidogenic acute regulatory protein (StAR), a rate-limiting component in steroidogenesis, to rhythmically increase its transcription [71]. These mechanisms concertedly drive glucocorticoids to peak at the early activity phase: in rodents, corticosterone peaks at 6:00–6:30 PM when the dark cycle starts [72]. In humans, cortisol reaches acrophase at 7:00–8:00 AM, and gradually declines until midnight (Figs. 1C, 2C) [73,74].
When rats experience 24-hour fasting, the corticosterone peak is delayed by 2 hours. Despite the delay, the peak magnitude remains unchanged [75]. Several TRF studies commonly reported that the peak time of corticosterone moved to the time when feeding started. Food availability only in 8:00 AM–4:00 PM for 2 weeks causes acrophase advance from 8:00 PM to noon in rats [76]. Another TRF trial allowing rats to eat only in 9:00–11:00 AM for 20 days also observed peak shift to 9:00 AM, when feeding started [77].
For humans, cortisol begins to increase immediately after fasting commenced (Fig. 1C) [78]. Five-day fasting increases cortisol levels and shifts the peak from the morning to the afternoon [79]. Other fasting experiments for 2.5 to 6 days dramatically elevates plasma cortisol levels [80–82]. Early TRF (feeding between 8:00 AM–2:00 PM) for 4 days slightly but significantly increases serum cortisol levels in the morning [83]. These results imply that intermittent fasting increases the level and frequency of cortisol secretion.
In addition to the aforementioned hormones, changes in other major hormones are covered in this section: growth hormone (GH), estradiol, melatonin, serotonin, and vaspin. However, as reports focusing on the effect of intermittent fasting are extremely limited for these hormones, we choose to briefly enumerate hormonal changes observed mainly in acute fasting.
GH is produced from the anterior pituitary gland and promotes the growth of lean body mass (bone and muscle) and lipolysis [84]. Pituitary GH secretion is mainly controlled by stimulatory growth hormone releasing hormone (GHRH) from the hypothalamus together with ghrelin and inhibitory somatostatin. Like other hormones, the SCN may be involved in the regulation of GH secretion. For example, Npy6r expression, enriched in the SCN, is required to maintain the normal level of GHRH expression from the hypothalamus, although its contribution to the circadian control of the GHRH–GH pathway remains unclear [85]. GH levels naturally peak at sleeping time: rodents at 9:00 AM–12:00 PM [86,87] and humans at 11:00 PM–2:00 AM [88,89]. Fasting for 6 hours in mice or for 72 hours in rats dramatically decreases plasma GH levels, which might be caused by decreased GHRH or increased somatostatin after food deprivation [90,91]. In humans, fasting for 37.5 hours elevates basal GH concentrations by 10-fold and reduces metabolic clearance rate of GH [92]. Other studies reported that the frequency of the GH cycle increased and GH peaks were observed even during daytime after 2- or 5-day fasting [93–95].
The hypothalamus-pituitary-gonad axis (HPG axis) controls the female reproductive cycle, the average period of which is 28 days in humans [96] and 4 days in rodents [97]. A 12-week ADF in rats disrupted the 4-day estrous cycle. Compared with control females, ADF rats show abnormally increased estradiol and significantly decreased luteinizing hormone [98]. Another ADF study in rats for 30 weeks also shows an increased risk of irregular or no estrous cycle [99]. Unlike ADF, 22-week TRF (feeding normal chow only at 9:00 PM–7:00 AM) isocaloric to ad libitum mice shows little effects on estradiol levels and rather improves reproductive function. This study suggests that TRF increases liver fibroblast growth factor 21, which stimulates gonadotropin-releasing hormone (GnRH) secretion from GnRH neurons [100].
Melatonin, derived from tryptophan, is secreted from the pineal gland and synchronizes the body to the central circadian cycle [101]. Melatonin starts to increase at the onset of darkness and peaks at 2:00–4:00 AM [102,103]. In humans, 3-day fasting causes acrophase advance by 81 minutes, but neither the maximal level nor the overall circadian pattern is changed [104]. During Ramadan fasting, the peak of plasma melatonin is lower but its circadian rhythm remains unchanged [105]. These might indicate that melatonin circadian rhythm is mainly regulated by the light/dark cycle rather than the feeding pattern.
Serotonin, as a hormone in blood, is mainly secreted from the enterochromaffin cells and stored in platelets. It exerts pleiotropic effects including vasoconstriction and smooth muscle contraction [106]. Serum serotonin levels exhibit circadian fluctuation: it peaks at 6:00–7:30 AM in humans [107,108] and in the afternoon in rodents [109]. After daytime TRF in rats for 3 weeks, the rhythmic pattern of serotonin in platelet-rich plasma is inversed, that is, the serotonin spikes at dawn instead [110]. Because this is not detected in platelet-free plasma, daytime TRF is thought to influence serotonin uptake and release from platelets.
Vaspin (visceral adipose tissue-derived serine protease inhibitor) attracts attention as an insulin-sensitizing adipokine [111]. Serum vaspin levels rise before meals and falls within 2 hours after eating. Furthermore, the circadian rhythm of vaspin shows a nocturnal rise reaching a peak at 6:00–7:00 AM and a trough at 3:00–4:00 PM in humans [112]. After 20-hour fasting, serum vaspin level is increased in humans [112].
Besides intermittent fasting in healthy subjects, a few studies evaluated its effect on patients with overt or subclinical metabolic diseases. For example, 5-week early TRF (6-hour feeding with dinner before 3:00 PM) was conducted in prediabetic subjects [113]. Although this study did not assess the 24-hour profile of insulin, the TRF regimen improved pancreatic β cell responsiveness to glucose as indicated by oral glucose tolerance test. However, another study testing the effect of 5:2 diet on type 2 diabetic patients over 12 weeks reported that this diet increased the risk of hypoglycemia [114]. Thus, the eligibility of intermittent fasting for diabetic patients should be carefully considered because glucose deprivation and induced ketogenesis can be detrimental to diabetic patients depending on the individual clinical condition [115,116].
A few studies evaluated the effect of intermittent fasting on thyroid abnormalities. In one study, 6-month ADF did not affect either free T4 or TSH in subclinical hypothyroidism subjects [117]. However, Ramadan studies suggested the need for higher doses of levothyroxine, a globally prescribed drug for hypothyroidism, in primary hypothyroidism patients because their serum TSH levels exceeded normal ranges after the Ramadan fast [118]. Therefore, patients with thyroid abnormalities should consult a physician about the dose and timing of drug intake during intermittent fasting.
Because of very limited research, it is difficult to assess the effect of intermittent fasting on other metabolic diseases such as adrenal insufficiency and Cushing syndrome. However, given the evidence regarding acute hormonal changes occurring immediately after fasting, future studies should further investigate the prolonged effects of intermittent fasting on hormonal status.
Limited food availability in specific periods stimulates an organism to alter the level and frequency of hormone secretion. To evaluate the efficacy of intermittent fasting, its effect on the endocrine system needs to be thoroughly examined. However, as such studies are scarce, it is difficult to conclude whether intermittent fasting is beneficial in aspect to hormonal changes. Nevertheless, this review aims to cover changes in the circadian rhythm of hormones after intermittent fasting in rodents (Table 1) and humans (Table 2). Furthermore, we organize the findings of several studies on early hormonal changes during acute fasting (Fig. 1) and present an estimate of hormonal changes induced by long-term intermittent fasting (Fig. 2).
In studying hormonal circadian rhythms, it is the interval between blood sampling that determines the accuracy and confidence of the experiments. In view of this, studies in rodents have an intrinsic limitation since frequent blood sampling is not feasible due to the small volume of total blood. On the other hand, research in human subjects can utilize blood samples collected at minute intervals, but usually the study pool is very small.
To clarify the effects and mechanisms of intermittent fasting and to examine whether we can recommend intermittent fasting regimes to patients with various metabolic diseases, further research needs to be conducted taking several factors into consideration. First, the analysis should discern the effect of intermittent fasting from that of reduced calorie intake, as calorie restriction itself has clinical implications. Reduced energy intake in certain types of intermittent fasting should be distinguished from isocaloric energy intake in TRF when analyzing the impact of intermittent feeding. Second, it seems that the coordination between feeding time and activity period of the organism is important, suggesting actimetry along with hormonal measurement as the gold standard in future research. In this point of view, it will be useful to study the results of dysregulated coordination between eating and activity patterns (i.e., night eating syndrome in humans). Third, the observed data should be translated with care to embrace possible interactions among hormonal systems as well as circadian fluctuations in the susceptibility of target organs. We speculate that well-designed studies will establish a better understanding of intermittent fasting and its effects on circadian hormonal regulation. This will enable the development of an efficient and safe intermittent fasting protocol with improved circadian hygiene.
ACKNOWLEDGMENTS
This work was supported by Research Resettlement Fund for the new faculty of Seoul National University (SNU), Creative-Pioneering Researchers Program through SNU, and the National Research Foundation of Korea (NRF) grants funded by the Korea government (MSIT) (No. 2020R1A4A3078962 and 2020R1-C1C1008033).
REFERENCES
1. Wyse CA, Biello SM, Gill JM. The bright-nights and dim-days of the urban photoperiod: implications for circadian rhythmicity, metabolism and obesity. Ann Med. 2014; 46:253–63.

2. Seaman DR. Weight gain as a consequence of living a modern lifestyle: a discussion of barriers to effective weight control and how to overcome them. J Chiropr Humanit. 2013; 20:27–35.

3. Patterson RE, Sears DD. Metabolic effects of intermittent fasting. Annu Rev Nutr. 2017; 37:371–93.

4. Rynders CA, Thomas EA, Zaman A, Pan Z, Catenacci VA, Melanson EL. Effectiveness of intermittent fasting and time-restricted feeding compared to continuous energy restriction for weight loss. Nutrients. 2019; 11:2442.

5. Harvie M, Howell A. Potential benefits and harms of intermittent energy restriction and intermittent fasting amongst obese, overweight and normal weight subjects: a narrative review of human and animal evidence. Behav Sci (Basel). 2017; 7:4.

6. Johnson JB, Summer W, Cutler RG, Martin B, Hyun DH, Dixit VD, et al. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic Biol Med. 2007; 42:665–74.

7. Harvie MN, Pegington M, Mattson MP, Frystyk J, Dillon B, Evans G, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond). 2011; 35:714–27.

8. Cignarella F, Cantoni C, Ghezzi L, Salter A, Dorsett Y, Chen L, et al. Intermittent fasting confers protection in CNS autoimmunity by altering the gut microbiota. Cell Metab. 2018; 27:1222–35.

9. Singh R, Lakhanpal D, Kumar S, Sharma S, Kataria H, Kaur M, et al. Late-onset intermittent fasting dietary restriction as a potential intervention to retard age-associated brain function impairments in male rats. Age (Dordr). 2012; 34:917–33.

10. Fontan-Lozano A, Saez-Cassanelli JL, Inda MC, de los Santos-Arteaga M, Sierra-Dominguez SA, Lopez-Lluch G, et al. Caloric restriction increases learning consolidation and facilitates synaptic plasticity through mechanisms dependent on NR2B subunits of the NMDA receptor. J Neurosci. 2007; 27:10185–95.

11. Longo VD, Panda S. Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab. 2016; 23:1048–59.

12. Mattson MP, de Cabo R. Effects of intermittent fasting on health, aging, and disease: reply. N Engl J Med. 2020; 382:1773–4.
13. Tinsley GM, La Bounty PM. Effects of intermittent fasting on body composition and clinical health markers in humans. Nutr Rev. 2015; 73:661–74.

14. Lowe DA, Wu N, Rohdin-Bibby L, Moore AH, Kelly N, Liu YE, et al. Effects of time-restricted eating on weight loss and other metabolic parameters in women and men with overweight and obesity: the TREAT randomized clinical trial. JAMA Intern Med. 2020; 180:1491–9.

15. Soeters MR, Lammers NM, Dubbelhuis PF, Ackermans M, Jonkers-Schuitema CF, Fliers E, et al. Intermittent fasting does not affect whole-body glucose, lipid, or protein metabolism. Am J Clin Nutr. 2009; 90:1244–51.

16. Attarzadeh Hosseini SR, Sardar MA, Hejazi K, Farahati S. The effect of Ramadan fasting and physical activity on body composition, serum osmolarity levels and some parameters of electrolytes in females. Int J Endocrinol Metab. 2013; 11:88–94.

17. Munoz-Hernandez L, Marquez-Lopez Z, Mehta R, Aguilar-Salinas CA. Intermittent fasting as part of the management for T2DM: from animal models to human clinical studies. Curr Diab Rep. 2020; 20:13.

18. Mrosovsky N, Reebs SG, Honrado GI, Salmon PA. Behavioural entrainment of circadian rhythms. Experientia. 1989; 45:696–702.

19. Pittendrigh CS. Temporal organization: reflections of a Darwinian clock-watcher. Annu Rev Physiol. 1993; 55:16–54.

20. Hastings MH, Maywood ES, Brancaccio M. Generation of circadian rhythms in the suprachiasmatic nucleus. Nat Rev Neurosci. 2018; 19:453–69.

22. Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012; 35:445–62.

24. Lee JH, Verma N, Thakkar N, Yeung C, Sung HK. Intermittent fasting: physiological implications on outcomes in mice and men. Physiology (Bethesda). 2020; 35:185–95.

25. Patterson RE, Laughlin GA, LaCroix AZ, Hartman SJ, Natarajan L, Senger CM, et al. Intermittent fasting and human metabolic health. J Acad Nutr Diet. 2015; 115:1203–12.

26. Wilcox G. Insulin and insulin resistance. Clin Biochem Rev. 2005; 26:19–39.
27. Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol Rev. 2018; 98:2133–223.

28. Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010; 466:627–31.

29. Perelis M, Marcheva B, Ramsey KM, Schipma MJ, Hutchison AL, Taguchi A, et al. Pancreatic β cell enhancers regulate rhythmic transcription of genes controlling insulin secretion. Science. 2015; 350:aac4250.

30. Vieira E, Burris TP, Quesada I. Clock genes, pancreatic function, and diabetes. Trends Mol Med. 2014; 20:685–93.

31. Qian J, Block GD, Colwell CS, Matveyenko AV. Consequences of exposure to light at night on the pancreatic islet circadian clock and function in rats. Diabetes. 2013; 62:3469–78.

32. Buijs RM, Chun SJ, Niijima A, Romijn HJ, Nagai K. Parasympathetic and sympathetic control of the pancreas: a role for the suprachiasmatic nucleus and other hypothalamic centers that are involved in the regulation of food intake. J Comp Neurol. 2001; 431:405–23.

33. Nyholm B, Walker M, Gravholt CH, Shearing PA, Sturis J, Alberti KG, et al. Twenty-four-hour insulin secretion rates, circulating concentrations of fuel substrates and gut incretin hormones in healthy offspring of type II (non-insulin-dependent) diabetic parents: evidence of several aberrations. Diabetologia. 1999; 42:1314–23.

34. Polonsky KS, Given BD, Van Cauter E. Twenty-four-hour profiles and pulsatile patterns of insulin secretion in normal and obese subjects. J Clin Invest. 1988; 81:442–8.

35. Ahren B. Diurnal variation in circulating leptin is dependent on gender, food intake and circulating insulin in mice. Acta Physiol Scand. 2000; 169:325–31.

36. Stenvers DJ, Scheer FAJL, Schrauwen P, la Fleur SE, Kalsbeek A. Circadian clocks and insulin resistance. Nat Rev Endocrinol. 2019; 15:75–89.

37. Dyar KA, Ciciliot S, Wright LE, Bienso RS, Tagliazucchi GM, Patel VR, et al. Muscle insulin sensitivity and glucose metabolism are controlled by the intrinsic muscle clock. Mol Metab. 2013; 3:29–41.

38. Gliniak CM, Brown JM, Noy N. The retinol-binding protein receptor STRA6 regulates diurnal insulin responses. J Biol Chem. 2017; 292:15080–93.

39. Duan W, Guo Z, Jiang H, Ware M, Mattson MP. Reversal of behavioral and metabolic abnormalities, and insulin resistance syndrome, by dietary restriction in mice deficient in brain-derived neurotrophic factor. Endocrinology. 2003; 144:2446–53.

40. Anson RM, Guo Z, de Cabo R, Iyun T, Rios M, Hagepanos A, et al. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc Natl Acad Sci U S A. 2003; 100:6216–20.

41. Rubin NH, Alinder G, Rietveld WJ, Rayford PL, Thompson JC. Restricted feeding schedules alter the circadian rhythms of serum insulin and gastric inhibitory polypeptide. Regul Pept. 1988; 23:279–88.

42. Klein S, Sakurai Y, Romijn JA, Carroll RM. Progressive alterations in lipid and glucose metabolism during short-term fasting in young adult men. Am J Physiol. 1993; 265(5 Pt 1):E801–6.

43. Heilbronn LK, Smith SR, Martin CK, Anton SD, Ravussin E. Alternate-day fasting in nonobese subjects: effects on body weight, body composition, and energy metabolism. Am J Clin Nutr. 2005; 81:69–73.

44. Harvie M, Wright C, Pegington M, McMullan D, Mitchell E, Martin B, et al. The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Br J Nutr. 2013; 110:1534–47.

45. Cienfuegos S, Gabel K, Kalam F, Ezpeleta M, Wiseman E, Pavlou V, et al. Effects of 4- and 6-h time-restricted feeding on weight and cardiometabolic health: a randomized controlled trial in adults with obesity. Cell Metab. 2020; 32:366–78.

46. Ikegami K, Refetoff S, Van Cauter E, Yoshimura T. Interconnection between circadian clocks and thyroid function. Nat Rev Endocrinol. 2019; 15:590–600.

47. Mullur R, Liu YY, Brent GA. Thyroid hormone regulation of metabolism. Physiol Rev. 2014; 94:355–82.

48. Fekete C, Lechan RM. Central regulation of hypothalamic-pituitary-thyroid axis under physiological and pathophysiological conditions. Endocr Rev. 2014; 35:159–94.

49. Kalsbeek A, Fliers E, Franke AN, Wortel J, Buijs RM. Functional connections between the suprachiasmatic nucleus and the thyroid gland as revealed by lesioning and viral tracing techniques in the rat. Endocrinology. 2000; 141:3832–41.

50. Aninye IO, Matsumoto S, Sidhaye AR, Wondisford FE. Circadian regulation of Tshb gene expression by Rev-Erbα (NR1D1) and nuclear corepressor 1 (NCOR1). J Biol Chem. 2014; 289:17070–7.

51. Jordan D, Rousset B, Perrin F, Fournier M, Orgiazzi J. Evidence for circadian variations in serum thyrotropin, 3,5, 3′-triiodothyronine, and thyroxine in the rat. Endocrinology. 1980; 107:1245–8.

52. Philippe J, Dibner C. Thyroid circadian timing: roles in physiology and thyroid malignancies. J Biol Rhythms. 2015; 30:76–83.
53. Russell W, Harrison RF, Smith N, Darzy K, Shalet S, Weetman AP, et al. Free triiodothyronine has a distinct circadian rhythm that is delayed but parallels thyrotropin levels. J Clin Endocrinol Metab. 2008; 93:2300–6.

54. de Vries EM, van Beeren HC, van Wijk AC, Kalsbeek A, Romijn JA, Fliers E, et al. Regulation of type 3 deiodinase in rodent liver and adipose tissue during fasting. Endocr Connect. 2020; 9:552–62.

55. Galton VA, Hernandez A, St Germain DL. The 5′-deiodinases are not essential for the fasting-induced decrease in circulating thyroid hormone levels in male mice: possible roles for the type 3 deiodinase and tissue sequestration of hormone. Endocrinology. 2014; 155:3172–81.

56. Boelen A, Wiersinga WM, Fliers E. Fasting-induced changes in the hypothalamus-pituitary-thyroid axis. Thyroid. 2008; 18:123–9.

57. Legradi G, Emerson CH, Ahima RS, Flier JS, Lechan RM. Leptin prevents fasting-induced suppression of prothyrotropin-releasing hormone messenger ribonucleic acid in neurons of the hypothalamic paraventricular nucleus. Endocrinology. 1997; 138:2569–76.

58. Lechan RM, Fekete C. Role of melanocortin signaling in the regulation of the hypothalamic-pituitary-thyroid (HPT) axis. Peptides. 2006; 27:310–25.

59. Guo F, Bakal K, Minokoshi Y, Hollenberg AN. Leptin signaling targets the thyrotropin-releasing hormone gene promoter in vivo. Endocrinology. 2004; 145:2221–7.

60. Ortiga-Carvalho TM, Curty FH, Nascimento-Saba CC, Moura EG, Polak J, Pazos-Moura CC. Pituitary neuromedin B content in experimental fasting and diabetes mellitus and correlation with thyrotropin secretion. Metabolism. 1997; 46:149–53.

61. Gardner DF, Kaplan MM, Stanley CA, Utiger RD. Effect of tri-iodothyronine replacement on the metabolic and pituitary responses to starvation. N Engl J Med. 1979; 300:579–84.

62. Merimee TJ, Fineberg ES. Starvation-induced alterations of circulating thyroid hormone concentrations in man. Metabolism. 1976; 25:79–83.

63. Stekovic S, Hofer SJ, Tripolt N, Aon MA, Royer P, Pein L, et al. Alternate day fasting improves physiological and molecular markers of aging in healthy, non-obese humans. Cell Metab. 2019; 30:462–76.

64. Moro T, Tinsley G, Bianco A, Marcolin G, Pacelli QF, Battaglia G, et al. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J Transl Med. 2016; 14:290.

65. Chung S, Son GH, Kim K. Circadian rhythm of adrenal glucocorticoid: its regulation and clinical implications. Biochim Biophys Acta. 2011; 1812:581–91.

66. Nicolaides NC, Charmandari E, Chrousos GP, Kino T. Circadian endocrine rhythms: the hypothalamic-pituitary-adrenal axis and its actions. Ann N Y Acad Sci. 2014; 1318:71–80.

67. Kim ER, Xu Y, Cassidy RM, Lu Y, Yang Y, Tian J, et al. Paraventricular hypothalamus mediates diurnal rhythm of metabolism. Nat Commun. 2020; 11:3794.

68. Zhu C, Xu Y, Jiang Z, Tian JB, Cassidy RM, Cai ZL, et al. Disrupted hypothalamic CRH neuron responsiveness contributes to diet-induced obesity. EMBO Rep. 2020; 21:e49210.
69. Buijs RM, Kalsbeek A. Hypothalamic integration of central and peripheral clocks. Nat Rev Neurosci. 2001; 2:521–6.

70. Ulrich-Lai YM, Arnhold MM, Engeland WC. Adrenal splanchnic innervation contributes to the diurnal rhythm of plasma corticosterone in rats by modulating adrenal sensitivity to ACTH. Am J Physiol Regul Integr Comp Physiol. 2006; 290:R1128–35.

71. Son GH, Chung S, Choe HK, Kim HD, Baik SM, Lee H, et al. Adrenal peripheral clock controls the autonomous circadian rhythm of glucocorticoid by causing rhythmic steroid production. Proc Natl Acad Sci U S A. 2008; 105:20970–5.

72. Kakihana R, Moore JA. Circadian rhythm of corticosterone in mice: the effect of chronic consumption of alcohol. Psychopharmacologia. 1976; 46:301–5.

74. Morris CJ, Aeschbach D, Scheer FA. Circadian system, sleep and endocrinology. Mol Cell Endocrinol. 2012; 349:91–104.

75. Bellinger LL, Mendel VE, Moberg GP. Circadian insulin, GH, prolactin, corticosterone and glucose rhythms in fed and fasted rats. Horm Metab Res. 1975; 7:132–5.

76. Morimoto Y, Arisue K, Yamamura Y. Relationship between circadian rhythm of food intake and that of plasma corticosterone and effect of food restriction on circadian adrenocortical rhythm in the rat. Neuroendocrinology. 1977; 23:212–22.

77. Wilkinson CW, Shinsako J, Dallman MF. Daily rhythms in adrenal responsiveness to adrenocorticotropin are determined primarily by the time of feeding in the rat. Endocrinology. 1979; 104:350–9.

78. Hojlund K, Wildner-Christensen M, Eshoj O, Skjaerbaek C, Holst JJ, Koldkjaer O, et al. Reference intervals for glucose, beta-cell polypeptides, and counterregulatory factors during prolonged fasting. Am J Physiol Endocrinol Metab. 2001; 280:E50–8.
79. Bergendahl M, Vance ML, Iranmanesh A, Thorner MO, Veldhuis JD. Fasting as a metabolic stress paradigm selectively amplifies cortisol secretory burst mass and delays the time of maximal nyctohemeral cortisol concentrations in healthy men. J Clin Endocrinol Metab. 1996; 81:692–9.

80. Johnstone AM, Faber P, Andrew R, Gibney ER, Elia M, Lobley G, et al. Influence of short-term dietary weight loss on cortisol secretion and metabolism in obese men. Eur J Endocrinol. 2004; 150:185–94.

81. Schurgin S, Canavan B, Koutkia P, Depaoli AM, Grinspoon S. Endocrine and metabolic effects of physiologic r-metHuLeptin administration during acute caloric deprivation in normal-weight women. J Clin Endocrinol Metab. 2004; 89:5402–9.

82. Veldhuis JD, Iranmanesh A, Evans WS, Lizarralde G, Thorner MO, Vance ML. Amplitude suppression of the pulsatile mode of immunoradiometric luteinizing hormone release in fasting-induced hypoandrogenemia in normal men. J Clin Endocrinol Metab. 1993; 76:587–93.

83. Jamshed H, Beyl RA, Della Manna DL, Yang ES, Ravussin E, Peterson CM. Early time-restricted feeding improves 24-hour glucose levels and affects markers of the circadian clock, aging, and autophagy in humans. Nutrients. 2019; 11:1234.

84. Lu M, Flanagan JU, Langley RJ, Hay MP, Perry JK. Targeting growth hormone function: strategies and therapeutic applications. Signal Transduct Target Ther. 2019; 4:3.

85. Yulyaningsih E, Loh K, Lin S, Lau J, Zhang L, Shi Y, et al. Pancreatic polypeptide controls energy homeostasis via Npy6r signaling in the suprachiasmatic nucleus in mice. Cell Metab. 2014; 19:58–72.

86. Steyn FJ, Huang L, Ngo ST, Leong JW, Tan HY, Xie TY, et al. Development of a method for the determination of pulsatile growth hormone secretion in mice. Endocrinology. 2011; 152:3165–71.

87. Bednarz K, Alshafie W, Aufmkolk S, Desserteaux T, Markam PS, Storch KF, et al. Ultradian secretion of growth hormone in mice: linking physiology with changes in synapse parameters using super-resolution microscopy. Front Neural Circuits. 2020; 14:21.

88. Takahashi Y, Kipnis DM, Daughaday WH. Growth hormone secretion during sleep. J Clin Invest. 1968; 47:2079–90.

89. Brandenberger G, Weibel L. The 24-h growth hormone rhythm in men: sleep and circadian influences questioned. J Sleep Res. 2004; 13:251–5.

90. Bruno JF, Olchovsky D, White JD, Leidy JW, Song J, Berelowitz M. Influence of food deprivation in the rat on hypothalamic expression of growth hormone-releasing factor and somatostatin. Endocrinology. 1990; 127:2111–6.

91. Huang L, Tan HY, Fogarty MJ, Andrews ZB, Veldhuis JD, Herzog H, et al. Actions of NPY, and its Y1 and Y2 receptors on pulsatile growth hormone secretion during the fed and fasted state. J Neurosci. 2014; 34:16309–19.

92. Moller L, Dalman L, Norrelund H, Billestrup N, Frystyk J, Moller N, et al. Impact of fasting on growth hormone signaling and action in muscle and fat. J Clin Endocrinol Metab. 2009; 94:965–72.

93. Ho KY, Veldhuis JD, Johnson ML, Furlanetto R, Evans WS, Alberti KG, et al. Fasting enhances growth hormone secretion and amplifies the complex rhythms of growth hormone secretion in man. J Clin Invest. 1988; 81:968–75.

94. Hartman ML, Veldhuis JD, Johnson ML, Lee MM, Alberti KG, Samojlik E, et al. Augmented growth hormone (GH) secretory burst frequency and amplitude mediate enhanced GH secretion during a two-day fast in normal men. J Clin Endocrinol Metab. 1992; 74:757–65.
95. Avram AM, Jaffe CA, Symons KV, Barkan AL. Endogenous circulating ghrelin does not mediate growth hormone rhythmicity or response to fasting. J Clin Endocrinol Metab. 2005; 90:2982–7.

96. Buffenstein R, Poppitt SD, McDevitt RM, Prentice AM. Food intake and the menstrual cycle: a retrospective analysis, with implications for appetite research. Physiol Behav. 1995; 58:1067–77.

97. Miller BH, Takahashi JS. Central circadian control of female reproductive function. Front Endocrinol (Lausanne). 2014; 4:195.

98. Kumar S, Kaur G. Intermittent fasting dietary restriction regimen negatively influences reproduction in young rats: a study of hypothalamo-hypophysial-gonadal axis. PLoS One. 2013; 8:e52416.

99. Martin B, Pearson M, Kebejian L, Golden E, Keselman A, Bender M, et al. Sex-dependent metabolic, neuroendocrine, and cognitive responses to dietary energy restriction and excess. Endocrinology. 2007; 148:4318–33.

100. Hua L, Feng B, Huang L, Li J, Luo T, Jiang X, et al. Time-restricted feeding improves the reproductive function of female mice via liver fibroblast growth factor 21. Clin Transl Med. 2020; 10:e195.

102. Uchida K, Okamoto N, Ohara K, Morita Y. Daily rhythm of serum melatonin in patients with dementia of the degenerate type. Brain Res. 1996; 717:154–9.

103. Selmaoui B, Touitou Y. Reproducibility of the circadian rhythms of serum cortisol and melatonin in healthy subjects: a study of three different 24-h cycles over six weeks. Life Sci. 2003; 73:3339–49.

104. Berga SL, Loucks TL, Cameron JL. Endocrine and chronobiological effects of fasting in women. Fertil Steril. 2001; 75:926–32.

105. Almeneessier AS, Bahammam AS, Sharif MM, Bahammam SA, Nashwan SZ, Pandi Perumal SR, et al. The influence of intermittent fasting on the circadian pattern of melatonin while controlling for caloric intake, energy expenditure, light exposure, and sleep schedules: a preliminary report. Ann Thorac Med. 2017; 12:183–90.

106. Mohammad-Zadeh LF, Moses L, Gwaltney-Brant SM. Serotonin: a review. J Vet Pharmacol Ther. 2008; 31:187–99.

107. Sauerbier I, von Mayersbach H. Circadian variation of serotonin levels in human blood. Horm Metab Res. 1976; 8:157–8.

108. Kwon O, Yu JH, Jeong E, Yoo HJ, Kim MS. Meal-related oscillations in the serum serotonin levels in healthy young men. Clin Endocrinol (Oxf). 2018; 88:549–55.

109. Sundar IK, Yao H, Huang Y, Lyda E, Sime PJ, Sellix MT, et al. Serotonin and corticosterone rhythms in mice exposed to cigarette smoke and in patients with COPD: implication for COPD-associated neuropathogenesis. PLoS One. 2014; 9:e87999.

110. Valdes-Fuentes M, Vera-Rivera G, De Ita-Perez D, Mendez I, Miranda MI, Diaz-Munoz M. Effect of daytime-restricted feeding in the daily variations of liver metabolism and blood transport of serotonin in rat. Physiol Rep. 2015; 3:e12389.

111. Hida K, Wada J, Eguchi J, Zhang H, Baba M, Seida A, et al. Visceral adipose tissue-derived serine protease inhibitor: a unique insulin-sensitizing adipocytokine in obesity. Proc Natl Acad Sci U S A. 2005; 102:10610–5.

112. Jeong E, Youn BS, Kim DW, Kim EH, Park JW, Namkoong C, et al. Circadian rhythm of serum vaspin in healthy male volunteers: relation to meals. J Clin Endocrinol Metab. 2010; 95:1869–75.

113. Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 2018; 27:1212–21.

114. Corley BT, Carroll RW, Hall RM, Weatherall M, Parry-Strong A, Krebs JD. Intermittent fasting in type 2 diabetes mellitus and the risk of hypoglycaemia: a randomized controlled trial. Diabet Med. 2018; 35:588–94.

115. Beta-blockers. Part II: the effect of associated disease states on the choice of a beta-blocker. Aust Nurses J. 1982; 11:31. 94.
116. Ahmed SH, Chowdhury TA, Hussain S, Syed A, Karamat A, Helmy A, et al. Ramadan and diabetes: a narrative review and practice update. Diabetes Ther. 2020; 11:2477–520.

Fig. 1
Graphs illustrating the relative levels of hormones during acute fasting in human (dashed line). Fasting started at different time points (an arrow head in each graph): (A) insulin, fasting onset at 6:00 PM [42], (B) triiodothyronine, fasting onset at 11:30 PM [61], (C) cortisol, fasting onset at 8:30 AM [78]. For comparison, the hormonal level during regular feeding (solid line; breakfast at 7:00 AM, lunch at 12:00 PM, and dinner at 6:00 PM) was depicted in accordance with the clock time. X axis means duration from fasting (hours).
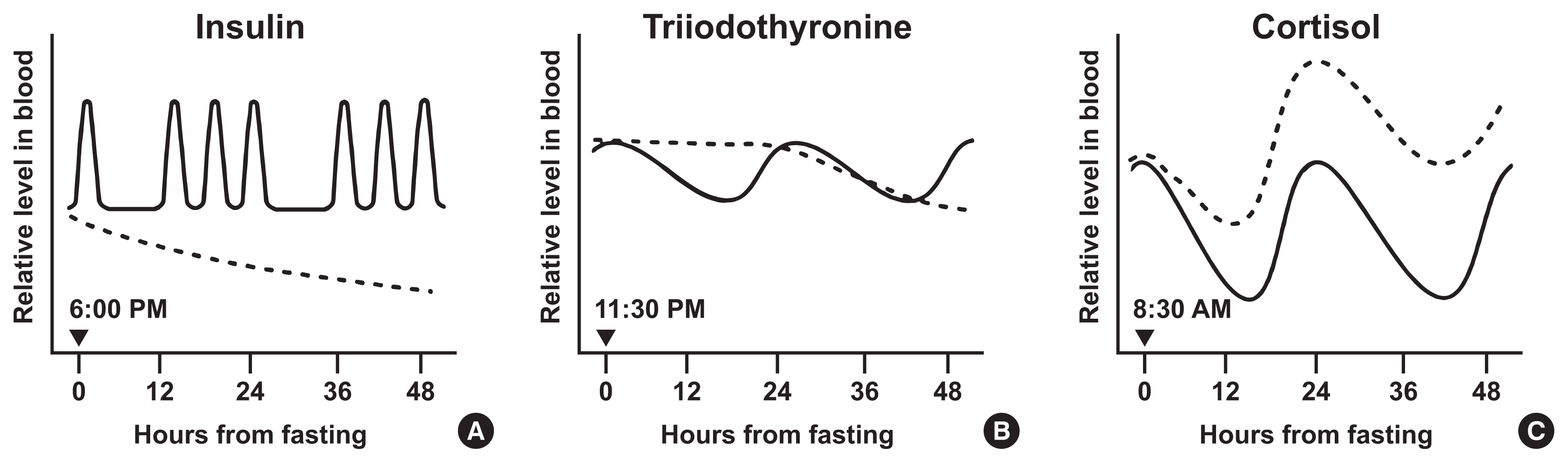
Fig. 2
Estimated changes in hormones after long-term intermittent fasting in human (dashed line). X axis presents daily light cycle: light box means daytime (6:00 AM–6:00 PM) and dark box indicates nighttime (6:00 PM–6:00 AM). Normal circadian rhythms of hormones were presented with solid line. (A) Insulin; postprandial peaks were depicted after breakfast (7:00 AM), lunch (12:00 PM), and dinner (6:00 PM). (B) Triiodothyronine. (C) Cortisol.
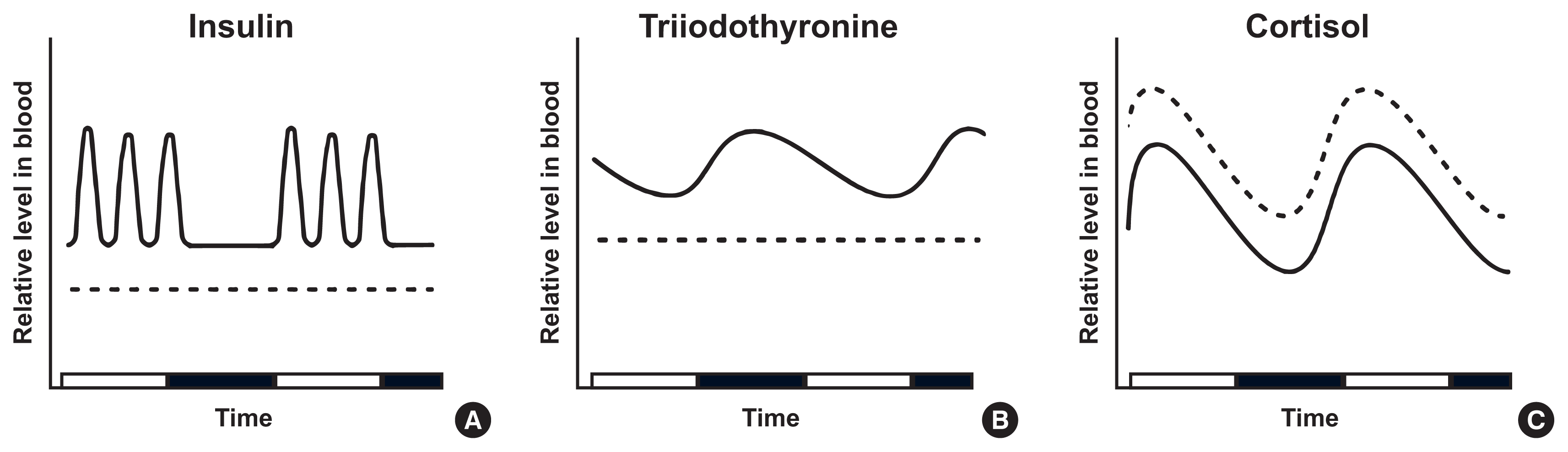
Table 1
Summary on the Changes in Hormonal Circadian Rhythm after Intermittent Fasting in Wildtype Rodents
| Hormone | Study | Strain (sex, n) | Type (duration) | Changes after intervention | |
|---|---|---|---|---|---|
|
|
|||||
| Mean level | Acrophase shift | ||||
| Insulin | Duan et al. (2003) [39] | C57BL/6 mouse (male, 8–10) | ADF (3 months) | ↓ | ND |
| Anson et al. (2003) [40] | C57BL/6 mouse (male, 8) | ADF (20 weeks) | ↓ | ND | |
| Rubin et al. (1988) [41] | Sprague-Dawley rat (male, 36 or 48) | TRF (3 weeks) | ↑ | 9:30 PM → 12:00–1:00 PM | |
|
|
|||||
| Corticosterone | Morimoto et al. (1977) [76]a | Sprague-Dawley rat (female, 4 in each sampling time) | TRF (14 days) | ND | 8:00 PM → 12:00 PM |
| Wilkinson et al. (1979) [77] | Sprague-Dawley rat (male, 6–8 in each sampling time) | TRF (20 days) | Unchanged | 9:00 PM → 9:00 AM | |
Table 2
Summary on the Changes in Hormonal Circadian Rhythm after Intermittent Fasting in Humans
| Hormone | Study | Subjects (sex, age, n) | Type (duration) | Changes after intervention | |
|---|---|---|---|---|---|
|
|
|||||
| Mean level | Acrophase shift | ||||
| Insulin | Heilbronn et al. (2005) [43] | Healthy, nonobese (men, 8; women, 8) | ADF (22 days) | ↓ | ND |
| Harvie et al. (2011) [7] | Overweight or obese (women, 42) | 5:2 diet (6 months) | ↓ | ND | |
| Harvie et al. (2013) [44] | Overweight (women, 37) | 5:2 diet (4 monthsa) | ↓ | ND | |
| Cienfuegos et al. (2020) [45] | Obese (4 hours TRF: 16, 6 hours TRF: 19) | TRF (8 weeks) | ↓ | ND | |
|
|
|||||
| Triiodothyronine | Stekovic et al. (2019) [63] | Healthy (4 weeks: 29, 6 months: 30) | ADF (4 weeks, 6 months) | ↓ | ND |
| Moro et al. (2016) [64] | Resistance-trained (men, 17) | TRF (8 weeks) | ↓ | ND | |
|
|
|||||
| Cortisol | Jamshed et al. (2019) [83] | Overweight (men, 7; women, 4) | TRF (4 days) | ↑ | ND |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download