Denosumab was approved for use in Korea in 2017, and its use has been rapidly increasing since it was classified as a reimbursable initial therapy for osteoporosis in April 2019. South Korea has a national health insurance system that enables all citizens to benefit from medical services with ease. Nonetheless, a problematic reality is that clinicians’ decision-making process in real-world practice is more directly affected by health insurance reimbursement policies than by medical guidelines.
The American Association of Clinical Endocrinology (AACE) and the Endocrine Society recommend denosumab as the initial treatment for patients at high or very high risk for osteoporotic fracture [1,2]. It has demonstrated superior efficacy for osteoporosis treatment, as a single subcutaneous injection of 60 mg once every 6 months reduces the incidence of vertebral fractures by 60%, nonvertebral fractures by 20%, and hip fractures by 40% over 3 years [3]. Moreover, the Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) extended study demonstrated that denosumab continued to increase bone density for 10 years [4].
However, this elevated bone density decreases rapidly after the discontinuation of denosumab. In FREEDOM extended study, the bone mineral density (BMD) that had risen for 10 years decreased sharply during just 1 year after discontinuation. Of particular note, femoral BMD decreased to an even lower level than baseline. This is well known to be due to the rebound of bone turnover markers after discontinuation of denosumab. During this rapid bone loss period, some patients have also been reported to experience rebound-associated fractures [4,5]. Rebound-associated fractures are not encountered in all patients; however, once they occur, they lead to multiple vertebral fractures, which can result in serious problems [6].
Rebound-associated fractures can be prevented by using other anti-resorptive agents [7]. In patients who received alendronate after discontinuation of denosumab, their BMD, which had been increased by the use of denosumab, was maintained or slightly increased [5,8]. Therefore, the European Calcified Tissue Society published a systematic review and position statement stating that denosumab should not be stopped without considering alternative treatments in order to prevent a rebound in bone turnover, rapid bone loss and an increased risk of fracture [9]. The Endocrine Society recommended that the administration of denosumab should not be delayed or stopped without subsequent antiresorptive (e.g., bisphosphonate, hormone therapy, or selective estrogen receptor modulator) or other therapy [1]. The AACE guideline provided a Grade A recommendation with a best evidence level of 1 stating that if denosumab therapy is discontinued, patients should be transitioned to another antiresorptive agent [2].
However, under the current Korean health insurance reimbursement criteria, no drug is reimbursable if a patient’s BMD improves to a T-score of ≥−2.5 through denosumab therapy. Since denosumab is currently frequently used, if the therapy is discontinued 2 to 3 years after initiation without subsequent pharmacologic treatment, many patients may suffer rebound-associated fractures. This possibility is worrisome from both socioeconomic and medical standpoints.
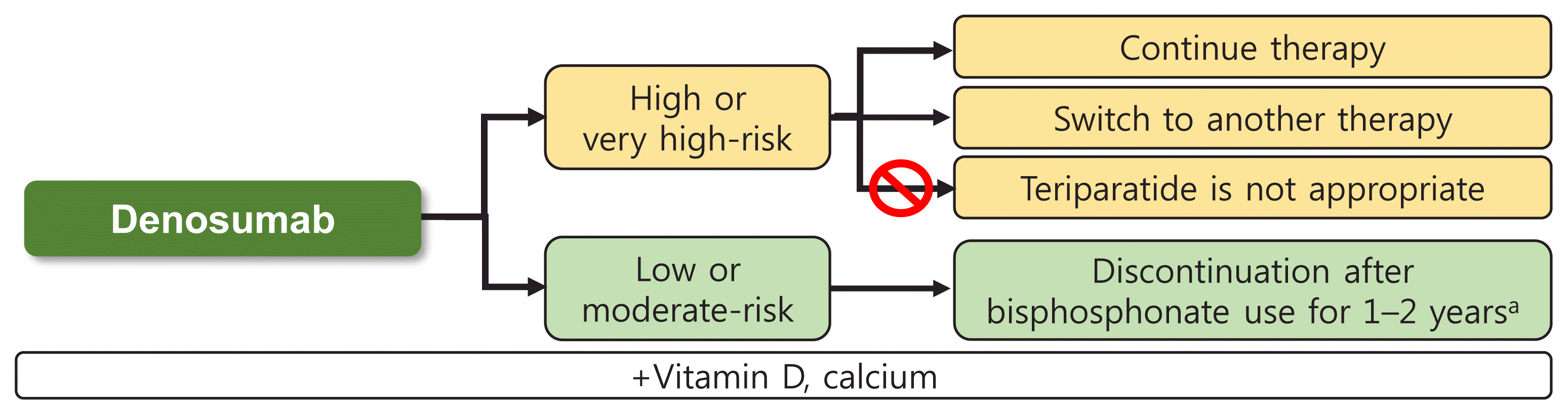
For patients who are still at high or very high risk for fracture despite denosumab treatment, romosozumab can be used [10]; however, medical insurance coverage is complicated in such cases, causing considerable difficulties from a practical perspective. In these cases, teriparatide is not appropriate [11]. The Insurance Committee of the Korean Endocrine Society summarized the above contents in Fig. 1.
Therefore, it is necessary to provide insurance coverage for antiresorptive agents for at least 1 year after discontinuation of denosumab treatment, regardless of BMD. Subsequent treatment should be provided after discontinuation of denosumab therapy in order to prevent rapid bone loss and rebound-associated fractures. Support from the Korean national insurance system is needed.
ACKNOWLEDGMENTS
This position statement was developed by the Health Insurance Committee of the Korean Endocrine Society in consultation with the Korean Society for Bone and Mineral Research and the Korean Society of Osteoporosis.
REFERENCES
1. Shoback D, Rosen CJ, Black DM, Cheung AM, Murad MH, Eastell R. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society guideline update. J Clin Endocrinol Metab. 2020; 105:dgaa048.

2. Camacho PM, Petak SM, Binkley N, Diab DL, Eldeiry LS, Farooki A, et al. American Association of Clinical Endocrinologists/American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2020 update. Endocr Pract. 2020; 26(Suppl 1):1–46.

3. Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009; 361:756–65.

4. Bone HG, Wagman RB, Brandi ML, Brown JP, Chapurlat R, Cummings SR, et al. 10 Years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017; 5:513–23.

5. Brown JP, Roux C, Torring O, Ho PR, Beck Jensen JE, Gilchrist N, et al. Discontinuation of denosumab and associated fracture incidence: analysis from the Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) trial. J Bone Miner Res. 2013; 28:746–52.

6. Niimi R, Kono T, Nishihara A, Hasegawa M, Kono T, Sudo A. Rebound-associated vertebral fractures after discontinuation of denosumab for the treatment of maxillitis. Osteoporos Int. 2018; 29:769–72.

7. Bone HG, Bolognese MA, Yuen CK, Kendler DL, Miller PD, Yang YC, et al. Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab. 2011; 96:972–80.

8. Kendler D, Chines A, Clark P, Ebeling PR, McClung M, Rhee Y, et al. Bone mineral density after transitioning from denosumab to alendronate. J Clin Endocrinol Metab. 2020; 105:e255–64.

9. Tsourdi E, Langdahl B, Cohen-Solal M, Aubry-Rozier B, Eriksen EF, Guanabens N, et al. Discontinuation of denosumab therapy for osteoporosis: a systematic review and position statement by ECTS. Bone. 2017; 105:11–7.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download