INTRODUCTION
METHODS
RESULTS
Concepts and main methods of economic evaluation
Table 1
Costs and outcomes as key elements in economic evaluation
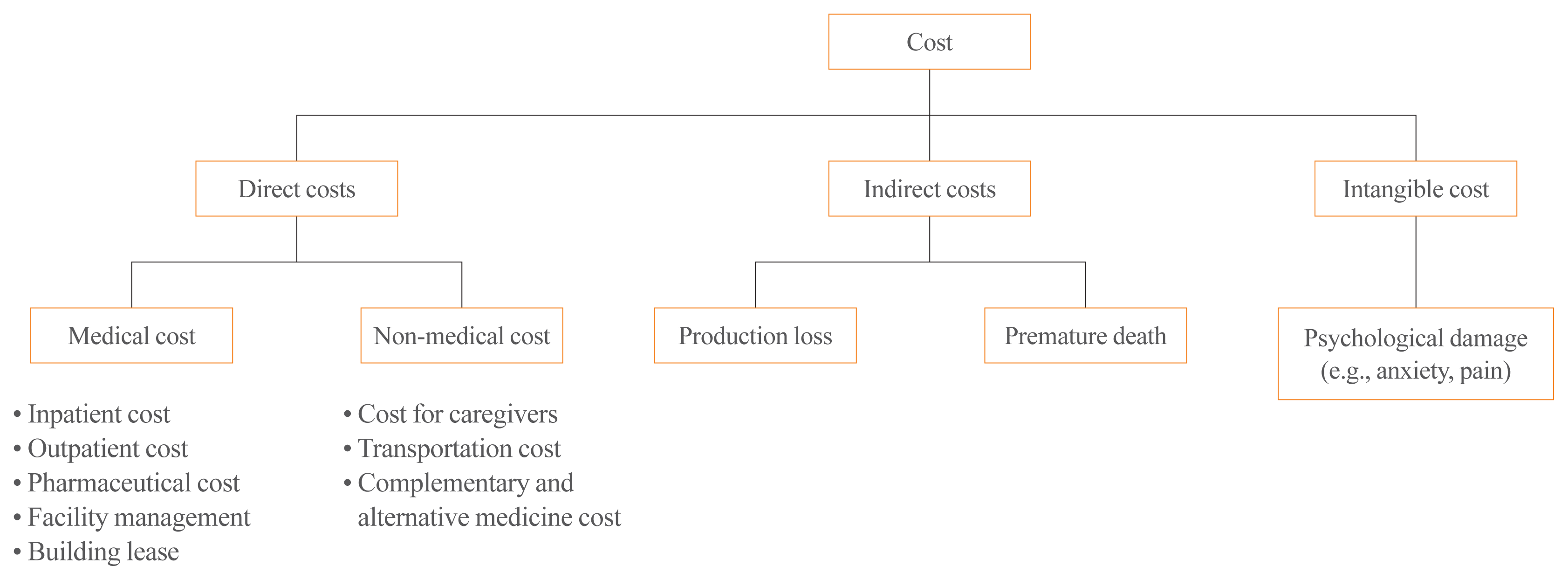
Costs
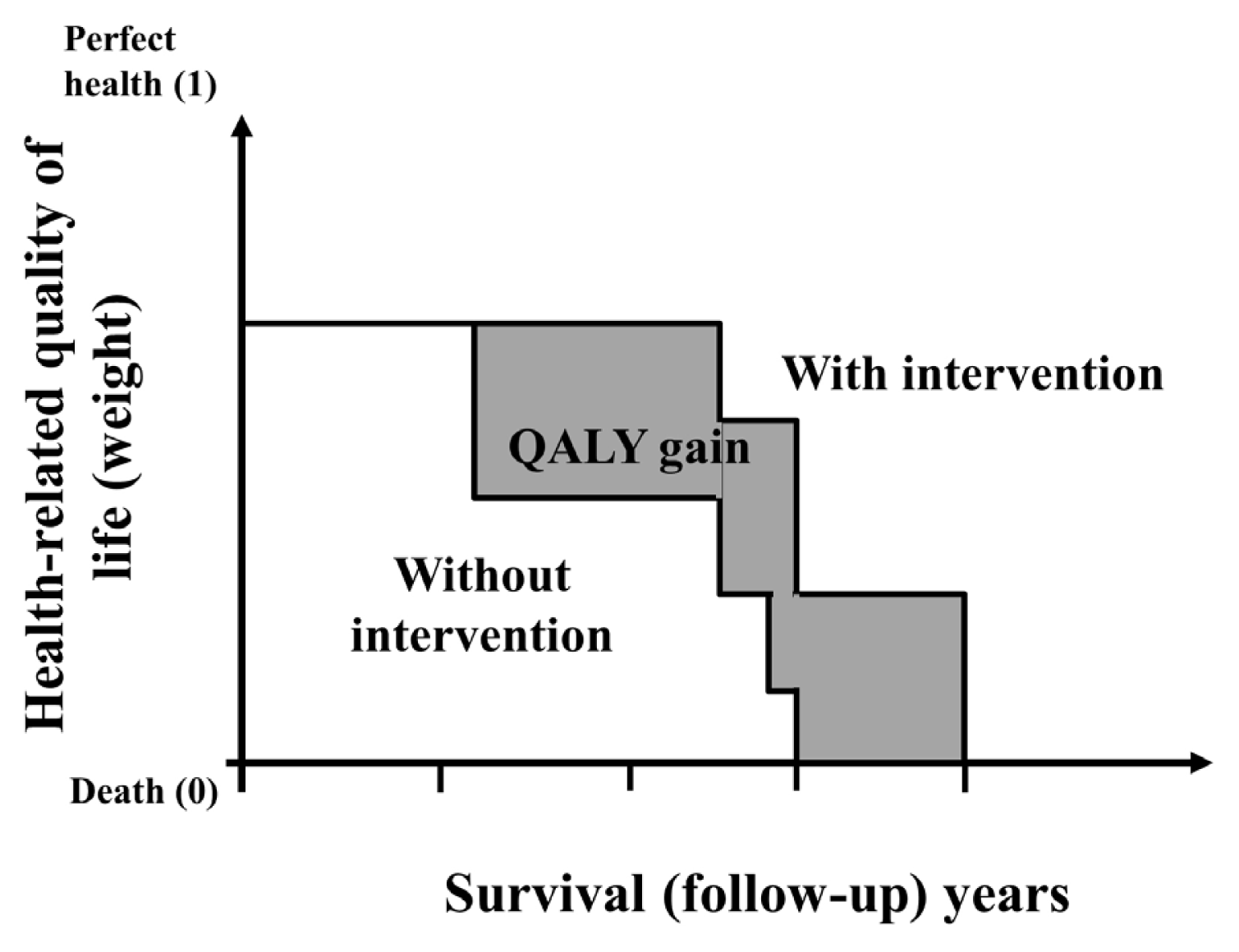
Outcomes
Examples of economic evaluation in previous thyroid cancer studies
Cost minimization analysis
Table 2
| Study | Country | Patients |
Intervention [I] Comparison [C] |
Direct cost [DC] Indirect cost [IC] |
Outcomes | Analysis | Results and conclusion |
|---|---|---|---|---|---|---|---|
| Lang et al. (2014) [26] | Hong Kong | 100,000 Hypothetical non-pregnant female patients in a cohort at age 50 years with low-risk PTC (1.5-cm cN0 PTC within one lobe) |
[I] TT with pCND [C] TT alone |
[DC] 20-Year cumulative cost, including procedural, complications, hospitalizations, annual routine surveillance (hospital costs published in the 2013 Government Gazette) [IC] Not included 3% discount rate |
Excess cost per case | Hypothetical model based on decision-tree design |
TT+pCND is more expensive in the medium- and long-term in low-risk PTC patients TT: USD 19,888.36 (20-year cumulative cost) TT: USD 19,888.36 (20-year cumulative cost) TT+pCND: USD 22,760.86 (20-year cumulative cost) Incremental cost: USD 2,872.50 Sensitivity analyses: no change |
| Shrime et al. (2007) [27] | Literature review from PubMed search 940 PTC citations published from January 1, 1966 to January 1, 2007 | 1 Million hypothetical low-risk PTC cases (age, metastasis, extent, and size of tumor [AMES] score <6, an AGES score <4, or adherence to the Memorial Sloan-Kettering low-risk categories) |
[I] HT [C] TT |
[DC] Hospital charges for inpatient operative procedure, follow-up costs, complication costs, and recurrence costs from Medicare [IC] Costs for death from unintentional injuries: $1,130,000 based on the National Safety Council estimates |
Cost and ICER for CSM or RFS CSM or RFS under the same cost assumption CSM and RFS were derived from a literature review (31 studies were finally selected) |
Monte Carlo microsimulation using the fixed probability estimates of complications and recurrence derived from the 31 eligible studies Sensitivity analyses using a 6% annual discount and cost-to-cost ratio estimated from the 31 eligible studies Threshold analyses: comparison of net monetary benefits obtained from TT and HT using a variable range of WTP values |
TT dominates HT as initial treatment for low-risk PTC 20-Year ICER for overall survival: [I] HT, $15,184; [C] TT, $13,979 20-Year ICER for RFS: [I] HT, $15,464; [C] TT, $20,005 TT is consistently cost-effective in Monte Carlo simulations Sensitivity analyses: HT was sensitive to recurrence rates and follow-up costs Threshold analyses: robust when compared with WTP |
| Guo et al. (2018) [28] | China | 256 PTC patients who underwent TT and BND |
[I] TT with simultaneous BND [C] TT with two-stage BND |
[DC] Hospital charges for surgery and related charges during hospital stays were obtained [IC] Accommodation, meals, and transportation fees |
Cost for DFS during a mean follow-up of 5 years |
Cost-effectiveness analysis was performed using a retrospective cohort. Sensitivity analyses: not done Threshold analyses: not done Discount rate was not applied |
Simultaneous BND was more cost-effective for the management of PTC patients than two-stage BND Two-stage BND was recommended in cases of internal jugular vein invasion 5-Year cost of surgery: simultaneous BND, $4,145.32; two-stage BND, $7,352.53 Cost per DFS: simultaneous BND, $4,423.34; two-stage BND, $8,158.25 |
| Walgama et al. (2020) [29] | USA | Hypothetical T2N0M0 PTC patients with undiscovered ipsilateral vocal fold paralysis. |
[I] Preoperative laryngoscopy prior to TT [C] No preoperative laryngoscopy prior to TT |
[DC] Medicare reimbursement data in Medicare from 2019 and the recent medical literature [IC] Not included 3% discount rate |
Cost-effectiveness measured by ICER (ΔCost/ΔQALY) Ipsilateral and contralateral vocal fold paralysis, as well as hypoparathyroidism over 30 years |
Decision tree model with literature-based probabilities Sensitivity analyses: varying the rate of VCP and selected variables. Monte Carlo simulation was performed to determine the overall uncertainty of the model. Threshold analyses: threshold of $100,000/QALY |
Fiberoptic laryngoscopy is cost-effective prior to TT in asymptomatic, low-risk thyroid cancer patients Preoperative laryngoscopy has an ICER of $45,193/QALY compared to no laryngoscopy Sensitivity analyses: laryngoscopy is cost-effective in 90.9% of cases Threshold analyses: the intervention is cost-effective if the incidence of vocal fold paralysis is at least 0.57%, or when the permissible rate of HT in cases of incidental paralysis is at least 41% |
| Kuo et al. (2018) [30] | USA | A euthyroid 40-year-old patient with an incidentally discovered thyroid nodule measuring 1 cm. The reference case did not have any clinical or radiologic evidence of cervical nodal metastases, neck surgery, radiation, or family history of thyroid cancer. |
[I] Ultrasound surveillance [C] Immediate biopsy |
[DC] Medicare reimbursement data in 2016, hospitalization cost data from the Nationwide Inpatient Sample, and a literature review. A 3% annual discount rate was applied to all future costs. [IC] Not included |
40-Year quality-adjusted life expectancy measured by ICER (ΔCost/ΔQALY) Effectiveness was measured in QALY. Each transition-state in the model was assigned a quality-adjustment factor based on literature and previous work. Event outcomes were natural history of thyroid nodules and thyroid cancer, test performance of thyroid FNA, and surgical outcomes of thyroidectomy and neck dissection. |
Markov transition-state model with probabilities obtained from the literature and expert opinion Sensitivity analyses: one-way and two-way sensitivity analyses and a Monte Carlo simulation were performed Threshold analyses: threshold of $100,000/QALYs |
US surveillance is more cost-effective than immediate biopsy for 1.0-cm thyroid nodules with an intermediate-suspicion sonographic pattern US surveillance was $1,829 less costly and 0.016 QALYs more effective than immediate biopsy Sensitivity analyses: immediate biopsy became cost-effective when the probability of malignancy increased from 15% to 84% or the cost of US increased from $129 to $793. Threshold analyses: robust when compared with WTP |
PTC, papillary thyroid cancer; TT, total thyroidectomy; pCND, prophylactic central neck dissection; USD, US dollar; AGES, Age Grade Extent Size; HT, hemi-thyroidectomy; ICER, incremental cost-effectiveness ratio; CSM, cause-specific mortality; RFS, recurrence-free survival; WTP, willingness to pay; BND, bilateral neck dissection; DFS, disease-free survival; QALY, quality-adjusted life-year; VCP, vocal cord paralysis; FNA, fine needle aspiration; US, United States.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download