1. Rossi GP, Bernini G, Calitype C, Desideri G, Fabris B, Ferri C, et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. 2006; 48:2293–300.

2. Kayser SC, Dekkers T, Groenewoud HJ, van der Wilt GJ, Carel Bakx J, van der Wel MC, et al. Study heterogeneity and estimation of prevalence of primary aldosteronism: a systematic review and meta-regression analysis. J Clin Endocrinol Metab. 2016; 101:2826–35.

3. Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016; 101:1889–916.

4. Monticone S, Viola A, Rossato D, Veglio F, Reincke M, Gomez-Sanchez C, et al. Adrenal vein sampling in primary aldosteronism: towards a standardised protocol. Lancet Diabetes Endocrinol. 2015; 3:296–303.

5. Umakoshi H, Tsuiki M, Yokomoto-Umakoshi M, Takeda Y, Takashi Y, Kurihara I, et al. Correlation between lateralization index of adrenal venous sampling and standardized outcome in primary aldosteronism. J Endocr Soc. 2018; 2:893–902.

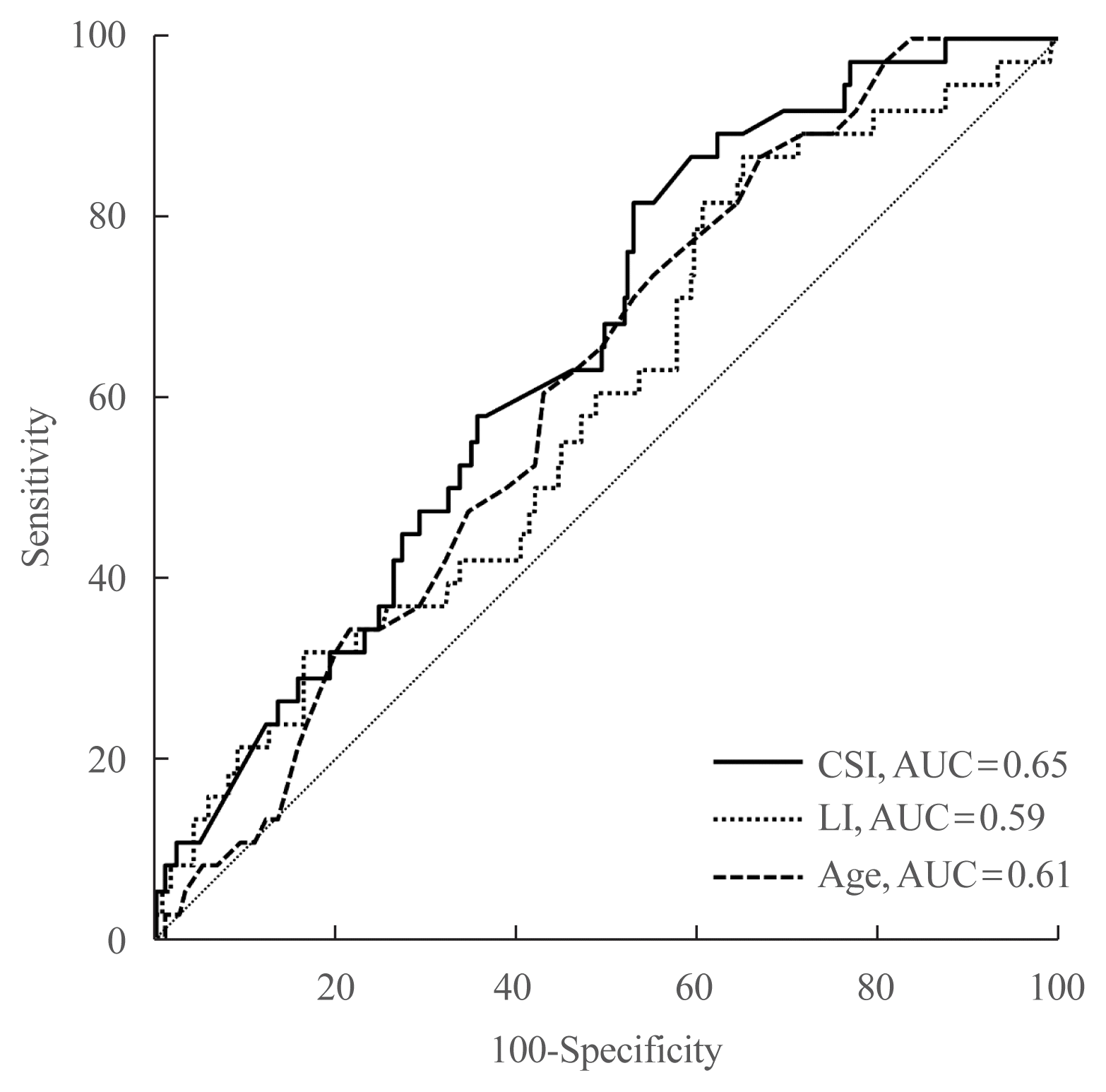
6. Tagawa M, Ghosn M, Wachtel H, Fraker D, Townsend RR, Trerotola S, et al. Lateralization index but not contralateral suppression at adrenal vein sampling predicts improvement in blood pressure after adrenalectomy for primary aldosteronism. J Hum Hypertens. 2017; 31:444–9.

7. Williams TA, Reincke M. Management of endocrine disease: diagnosis and management of primary aldosteronism: the Endocrine Society guideline 2016 revisited. Eur J Endocrinol. 2018; 179:R19–29.

8. Wolley MJ, Gordon RD, Ahmed AH, Stowasser M. Does contralateral suppression at adrenal venous sampling predict outcome following unilateral adrenalectomy for primary aldosteronism?: a retrospective study. J Clin Endocrinol Metab. 2015; 100:1477–84.

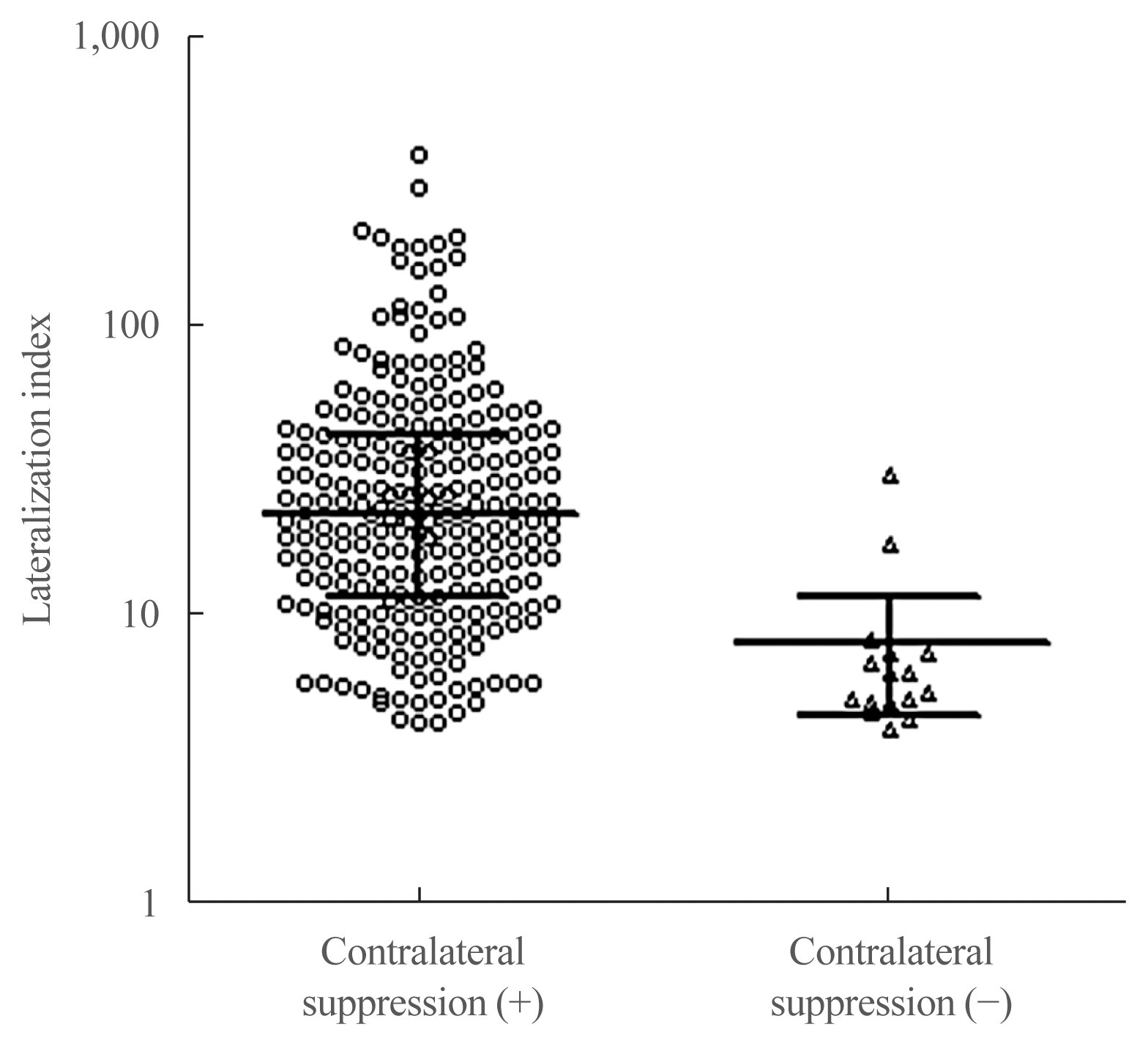
9. Umakoshi H, Tanase-Nakao K, Wada N, Ichijo T, Sone M, Inagaki N, et al. Importance of contralateral aldosterone suppression during adrenal vein sampling in the subtype evaluation of primary aldosteronism. Clin Endocrinol (Oxf). 2015; 83:462–7.

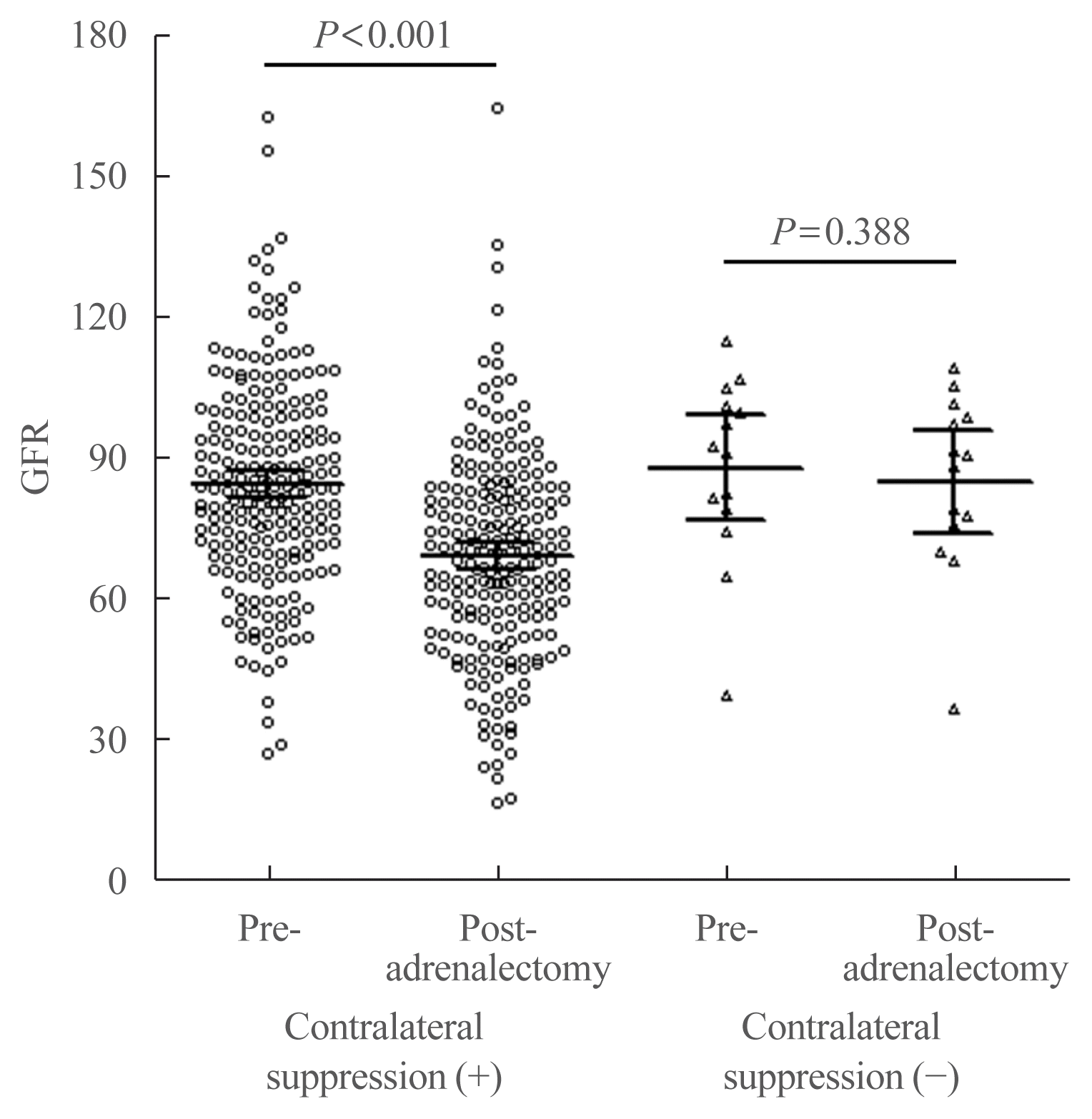
10. Monticone S, Satoh F, Viola A, Fischer E, Vonend O, Bernini G, et al. Aldosterone suppression on contralateral adrenal during adrenal vein sampling does not predict blood pressure response after adrenalectomy. J Clin Endocrinol Metab. 2014; 99:4158–66.

11. Shin SY, Park YR, Shin Y, Choi HJ, Park J, Lyu Y, et al. A de-identification method for bilingual clinical texts of various note types. J Korean Med Sci. 2015; 30:7–15.

12. Williams TA, Lenders JW, Mulatero P, Burrello J, Rottenkolber M, Adolf C, et al. Outcomes after adrenalectomy for unilateral primary aldosteronism: an international consensus on outcome measures and analysis of remission rates in an international cohort. Lancet Diabetes Endocrinol. 2017; 5:689–99.

13. Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Renal outcomes in medically and surgically treated primary aldosteronism. Hypertension. 2018; 72:658–66.

14. Chen ZW, Hung CS, Wu VC, Lin YH. TAIPAI Study Group. Primary aldosteronism and cerebrovascular diseases. Endocrinol Metab (Seoul). 2018; 33:429–34.

15. Sechi LA, Colussi G, Di Fabio A, Catena C. Cardiovascular and renal damage in primary aldosteronism: outcomes after treatment. Am J Hypertens. 2010; 23:1253–60.

16. Sechi LA, Novello M, Lapenna R, Baroselli S, Nadalini E, Colussi GL, et al. Long-term renal outcomes in patients with primary aldosteronism. JAMA. 2006; 295:2638–45.

17. Sechi LA, Di Fabio A, Bazzocchi M, Uzzau A, Catena C. Intrarenal hemodynamics in primary aldosteronism before and after treatment. J Clin Endocrinol Metab. 2009; 94:1191–7.

18. Park KS, Kim JH, Yang YS, Hong AR, Lee DH, Moon MK, et al. Outcomes analysis of surgical and medical treatments for patients with primary aldosteronism. Endocr J. 2017; 64:623–32.

19. Park KS, Kim JH, Ku EJ, Hong AR, Moon MK, Choi SH, et al. Clinical risk factors of postoperative hyperkalemia after adrenalectomy in patients with aldosterone-producing adenoma. Eur J Endocrinol. 2015; 172:725–31.

20. Shariq OA, Bancos I, Cronin PA, Farley DR, Richards ML, Thompson GB, et al. Contralateral suppression of aldosterone at adrenal venous sampling predicts hyperkalemia following adrenalectomy for primary aldosteronism. Surgery. 2018; 163:183–90.

21. Tahir A, McLaughlin K, Kline G. Severe hyperkalemia following adrenalectomy for aldosteronoma: prediction, pathogenesis and approach to clinical management: a case series. BMC Endocr Disord. 2016; 16:43.

22. Sukor N, Gordon RD, Ku YK, Jones M, Stowasser M. Role of unilateral adrenalectomy in bilateral primary aldosteronism: a 22-year single center experience. J Clin Endocrinol Metab. 2009; 94:2437–45.

23. Omata K, Satoh F, Morimoto R, Ito S, Yamazaki Y, Nakamura Y, et al. Cellular and genetic causes of idiopathic hyperaldosteronism. Hypertension. 2018; 72:874–80.

24. Rossitto G, Amar L, Azizi M, Riester A, Reincke M, Degenhart C, et al. Subtyping of primary aldosteronism in the avis-2 study: assessment of selectivity and lateralization. J Clin Endocrinol Metab. 2020; 105:dgz017.

25. Rossi GP, Seccia TM, Pessina AC. A diagnostic algorithm: the holy grail of primary aldosteronism. Nat Rev Endocrinol. 2011; 7:697–9.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download