Abstract
Background
Methods
Results
Conclusion
Notes
AUTHOR CONTRIBUTIONS
Conception or design: D.T.W.L., K.S.L.L. Acquisition, analysis, or interpretation of data: D.T.W.L., C.H.L., W.S.C., A.C.H.L., A.R.T., C.H.Y.F., C.Y.L., E.K.H.L., K.S.L.L. Drafting the work or revising: D.T.W.L., C.H.L., W.S.C., A.C.H.L., K.K.W.T., K.C.B.T., Y.C.W., C.W.L., I.F.N.H., K.S.L.L. Final approval of the manuscript: D.T.W.L., C.H.L., W.S.C., A.C.H.L., A.R.T., C.H.Y.F., C.Y.L., E.K.H.L., K.K.W.T., K.C.B.T., Y.C.W., C.W.L., I.F.N.H., K.S.L.L.
ACKNOWLEDGMENTS
REFERENCES
Fig. 1
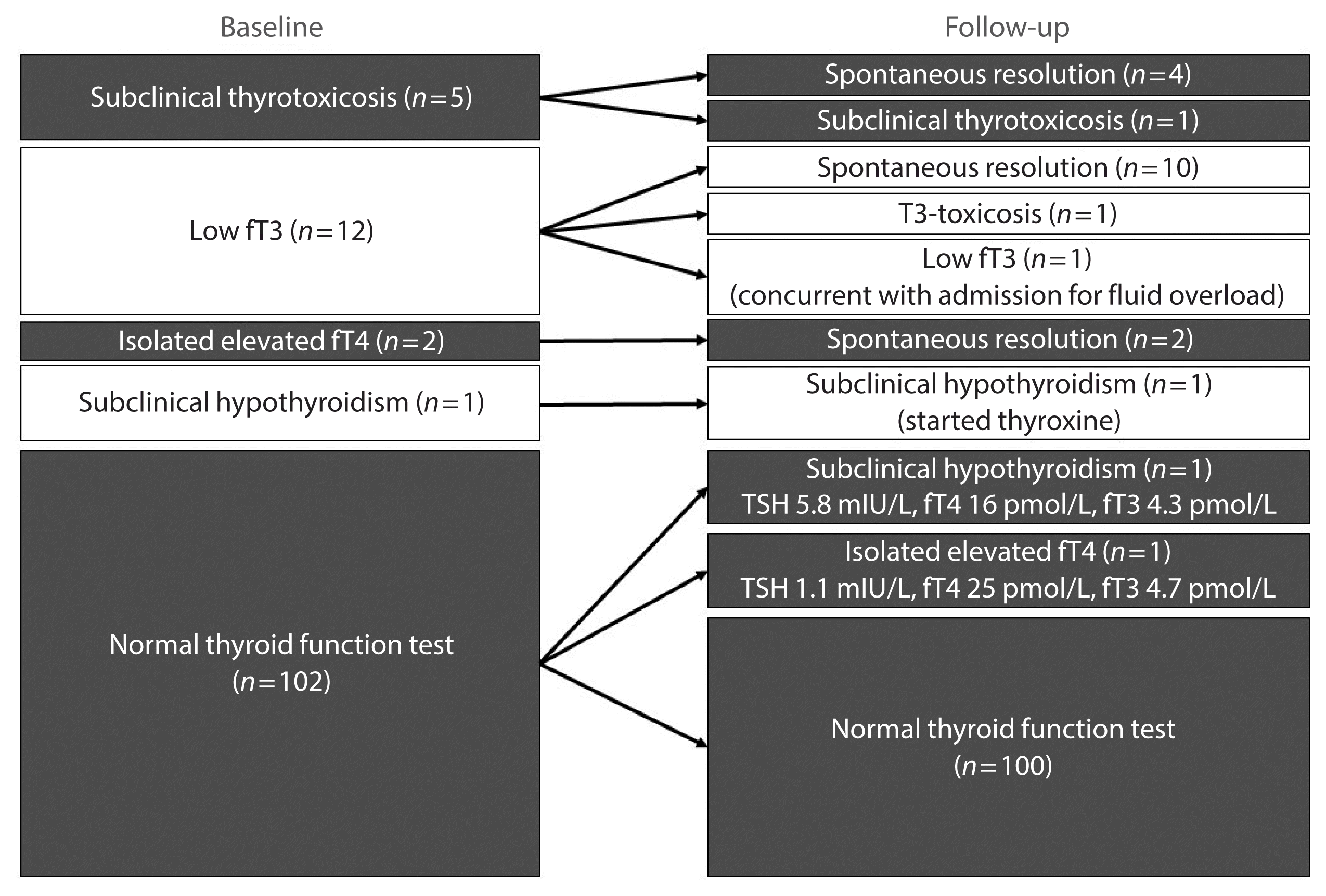
Table 1
Table 2
| Patient no. | On admission | COVID-19 treatment | Reassessment | Remarks | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||||||
| TSH, mIU/La | fT4, pmol/La | fT3, pmol/La | Anti-TPO, Ub | Anti-Tg, Ub | Anti-TSHR, IU/Lb | Days | TSH, mIU/La | fT4, pmol/La | fT3, pmol/La | Anti-TPO, Ub | Anti-Tg, Ub | Anti-TSHR, IU/Lb | |||
| 1 | <0.01c | 22 | 5.2 | 627.76c | 4.12 | 9.9c | IFN+RIB | 88 | 0.99 | 13 | 4.4 | 525.95c | 5.12 | 4.2c | Spontaneous resolution |
|
|
|||||||||||||||
| 2 | 0.06c | 16 | 3.8 | 17.01 | 4.98 | 0.9 | IFN+RIB | 27 | 0.70 | 21 | 3.7 | NA | NA | NA | Spontaneous resolution |
|
|
|||||||||||||||
| 3 | 0.24c | 17 | 3.2 | 10.25 | 18.52 | 0.5 | IFN+RIB | 99 | 0.86 | 20 | 4.3 | 17.79 | 19.69 | 1.0 | Spontaneous resolution |
|
|
|||||||||||||||
| 4 | 0.26c | 22 | 3.4 | 10.08 | 3.80 | 0.8 | IFN+RIB | 92 | 0.31c | 23 | 5.1 | 10.04 | 5.74 | 1.5c | Resolved to normal 3 weeks later |
|
|
|||||||||||||||
| 5 | 0.31c | 20 | 5.4 | 25.34 | 6.77 | 1.2c | IFN+RIB | 95 | 0.12c | 17 | 5.5 | 27.92 | 7.05 | 1.3c | Thyroid ultrasonography: unremarkable echogenicity and vascularity |
|
|
|||||||||||||||
| 6 | 0.53 | 20 | 2.8c | 33.13 | 4.19 | 0.6 | IFN+RIB | 89 | 0.64 | 18 | 3.9 | 32.99 | 6.33 | 1.0 | |
|
|
|||||||||||||||
| 7 | 0.55 | 16 | 2.9c | 151.02c | 2,957.1c | NA | IFN+RIB+DEX | 102 | 0.02c | 21 | 7.2 | 100.12c | 2,793.9c | 0.8 | Pending thyroid ultrasonography and nuclear scan |
|
|
|||||||||||||||
| 8 | 0.56 | 17 | 2.9c | 27.05 | 3.73 | 0.1 | IFN+RIB+DEX | 111 | 3.4 | 20 | 4.9 | 21.12 | 6.31 | 1.2c | |
|
|
|||||||||||||||
| 9 | 0.60 | 16 | 3.0c | 113.31c | 4.10 | 1.2c | IFN+RIB | 92 | 1.3 | 19 | 5.1 | 145.85c | 6.62 | 0.8 | |
|
|
|||||||||||||||
| 10 | 0.83 | 15 | 2.3c | 13.91 | 3.63 | 0.4 | IFN+RIB+DEX | 52 | 1.4 | 11c | 2.1c | NA | NA | NA | Admitted for fluid overload on reassessment |
|
|
|||||||||||||||
| 11 | 1.0 | 14 | 2.9c | 28.98 | 9.26 | 0.9 | IFN+RIB+DEX | 97 | 2.3 | 14 | 4.8 | 55.33 | 12.15 | 0.6 | |
|
|
|||||||||||||||
| 12 | 1.1 | 24 | 3.0c | 18.48 | 11.32 | 1.2c | None | 81 | 2.6 | 20 | 3.5 | 19.22 | 11.99 | 1.4c | |
|
|
|||||||||||||||
| 13 | 1.2 | 14 | 2.8c | 51.56 | 9.67 | 1.0 | IFN+RIB+DEX | 74 | 2.1 | 19 | 5.2 | 50.67 | 10.51 | 1.1c | |
|
|
|||||||||||||||
| 14 | 1.6 | 14 | 3.1c | 65.48 | 26.26 | 0.2 | None | 15 | 1.3 | 18 | 4.7 | NA | NA | NA | |
|
|
|||||||||||||||
| 15 | 1.7 | 15 | 3.1c | 48.13 | 4.33 | 0.7 | IFN+RIB | 47 | 1.3 | 14 | 3.9 | NA | NA | NA | |
|
|
|||||||||||||||
| 16 | 1.8 | 13 | 2.8c | 1,579.9c | 16.33 | 0.3 | IFN+RIB | 90 | 1.4 | 13 | 4.2 | 977.35c | 11.65 | 0.6 | |
|
|
|||||||||||||||
| 17 | 1.9 | 14 | 2.9 | 19.83 | 4.48 | 0.8 | IFN+RIB | 63 | 2.9 | 13 | 4.1 | 25.88 | 10.00 | 1.5 | |
|
|
|||||||||||||||
| 18 | 2.6 | 24c | 4.0 | 32.44 | 257.5c | 0.6 | IFN+RIB | 21 | 1.8 | 17 | 4.4 | NA | NA | NA | |
|
|
|||||||||||||||
| 19 | 2.8 | 24c | 4.4 | 42.46 | 4.12 | 1.3c | IFN+RIB | 93 | 2.1 | 22 | 4.2 | 60.84 | 6.34 | 0.9 | |
|
|
|||||||||||||||
| 20 | 11c | 12 | 3.9 | 18,719c | 177.1c | NA | IFN+RIB | 47 | 10c | 12 | NA | NA | NA | NA | Started thyroxine replacement |
TSH, thyroid-stimulating hormone; fT4, free thyroxine; fT3, free triiodothyronine; TPO, thyroid peroxidase; Tg, thyroglobulin; TSHR, TSH receptor antibody; COVID-19, coronavirus disease 2019; IFN, interferon beta-1b; RIB, ribavirin; NA, not available; DEX, dexamethasone.
Table 3
| Patient no. | On admission | COVID-19 treatment | Reassessment | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||||
| TSH, mIU/La | fT4, pmol/La | fT3, pmol/La | Anti-TPO, Ub | Anti-Tg, Ub | Anti-TSHR, IU/Lb | Days | TSH, mIU/La | fT4, pmol/La | fT3, pmol/La | Anti-TPO, Ub | Anti-Tg, Ub | Anti-TSHR, IU/Lb | ||
| 21 | 1.2 | 17 | 3.5 | 6.85 | 4.90 | 1.6c | IFN+RIB | 93 | 2.8 | 20 | 5.0 | 141.24c | 8.31 | 1.2c |
|
|
||||||||||||||
| 22 | 1.3 | 18 | NA | 45.45 | 4.31 | 1.0 | IFN+RIB | 89 | 1.7 | 17 | 5.3 | 148.21c | 6.58 | 1.4c |
|
|
||||||||||||||
| 23 | 2.2 | 16 | 4.6 | 97.06 | 4.84 | 0.2 | IFN+RIB | 90 | 1.7 | 20 | 5.6 | 122.37c | 7.31 | 1.2c |
|
|
||||||||||||||
| 24 | 1.1 | 17 | 4.4 | 99.85 | 6.62 | 0.7 | IFN+RIB | 105 | 0.47 | 19 | 5.2 | 128.51c | 9.67 | 0.7 |
TSH, thyroid-stimulating hormone; fT4, free thyroxine; fT3, free triiodothyronine; TPO, thyroid peroxidase; Tg, thyroglobulin; TSHR, TSH receptor antibody; COVID-19, coronavirus disease 2019; IFN, interferon beta-1b; RIB, ribavirin.
Table 4
| Variable | Significant increase in anti-TPO titer | No significant increase in anti-TPO titer | P value |
|---|---|---|---|
| Number | 16 | 66 | - |
|
|
|||
| Baseline characteristics | |||
| Age, yr | 61 (49–65) | 55 (41–63) | 0.429 |
| Male sex | 9 (56.3) | 30 (45.5) | 0.436 |
| Smoking | 2/12 (16.7) | 8/61 (13.1) | 0.665 |
| Drinking | 4/11 (36.4) | 11/60 (18.3) | 0.228 |
| TSH, mIU/L | 1.1 (0.9–1.8) | 1.1 (0.8–1.5) | 0.631 |
| fT4, pmol/L | 18 (17–19) | 18 (17–20) | 0.554 |
| fT3, pmol/L | 3.9 (3.6–4.4) | 4.1 (3.6–4.5) | 0.676 |
| Baseline anti-TPO titer, U | 43 (22–54) | 18 (10–33) | 0.005a |
| COVID-19 severity | 0.018a | ||
| Mild | 11 (68.8) | 55 (83.3) | |
| Moderate | 2 (12.5) | 11 (16.7) | |
| Severe | 3 (18.8) | 0 | |
|
|
|||
| Clinical course | |||
| Elevated CRP during hospitalization | 15 (93.8) | 44 (66.7) | 0.033a |
| Peak ESR during hospitalization, mm/hr | 68 (41–96) | 56 (35–93) | 0.564 |
| Length of hospitalization, day | 8 (6–11) | 8 (6–11) | 0.874 |
| Interferon beta-1b treatment | 14 (87.5) | 51 (77.3) | 0.503 |
| Dexamethasone requirement | 2 (12.5) | 7 (10.6) | 0.999 |
| Oxygen requirement | 2 (12.5) | 6 (9.1) | 0.650 |
| Intensive care unit admission | 1 (6.3) | 1 (1.5) | 0.354 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download