1. Lee JH, Song RY, Yi JW, Yu HW, Kwon H, Kim SJ, et al. Case-control study of papillary thyroid carcinoma on urinary and dietary iodine status in South Korea. World J Surg. 2018; 42:1424–31.

2. Lee JH, Hwang Y, Song RY, Yi JW, Yu HW, Kim SJ, et al. Relationship between iodine levels and papillary thyroid carcinoma: a systematic review and meta-analysis. Head Neck. 2017; 39:1711–8.

3. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Tobacco smoke and involuntary smoking. IARC Monogr Eval Carcinog Risks Hum. 2004; 83:1–1438.
4. Sokic SI, Adanja BJ, Vlajinac HD, Jankovic RR, Marinkovic JP, Zivaljevic VR. Risk factors for thyroid cancer. Neoplasma. 1994; 41:371–4.
5. Iribarren C, Haselkorn T, Tekawa IS, Friedman GD. Cohort study of thyroid cancer in a San Francisco Bay area population. Int J Cancer. 2001; 93:745–50.

6. Guignard R, Truong T, Rougier Y, Baron-Dubourdieu D, Guenel P. Alcohol drinking, tobacco smoking, and anthropometric characteristics as risk factors for thyroid cancer: a countrywide case-control study in New Caledonia. Am J Epidemiol. 2007; 166:1140–9.

7. Mack WJ, Preston-Martin S, Dal Maso L, Galanti R, Xiang M, Franceschi S, et al. A pooled analysis of case-control studies of thyroid cancer: cigarette smoking and consumption of alcohol, coffee, and tea. Cancer Causes Control. 2003; 14:773–85.
8. Kitahara CM, Linet MS, Beane Freeman LE, Check DP, Church TR, Park Y, et al. Cigarette smoking, alcohol intake, and thyroid cancer risk: a pooled analysis of five prospective studies in the United States. Cancer Causes Control. 2012; 23:1615–24.

9. Paquet M, Shivappa N, Hebert JR, Baron-Dubourdieu D, Boutron-Ruault MC, Guenel P, et al. Dietary inflammatory index and differentiated thyroid carcinoma risk: a population-based case-control study in New Caledonia. Am J Epidemiol. 2020; 189:95–107.

10. Truong T, Rougier Y, Dubourdieu D, Guihenneuc-Jouyaux C, Orsi L, Hemon D, et al. Time trends and geographic variations for thyroid cancer in New Caledonia, a very high incidence area (1985–1999). Eur J Cancer Prev. 2007; 16:62–70.

11. Truong T, Baron-Dubourdieu D, Rougier Y, Guenel P. Role of dietary iodine and cruciferous vegetables in thyroid cancer: a countrywide case-control study in New Caledonia. Cancer Causes Control. 2010; 21:1183–92.

12. Tcheandjieu C, Lesueur F, Sanchez M, Baron-Dubourdieu D, Guizard AV, Mulot C, et al. Fine-mapping of two differentiated thyroid carcinoma susceptibility loci at 9q22.33 and 14q13.3 detects novel candidate functional SNPs in Europeans from metropolitan France and Melanesians from New Caledonia. Int J Cancer. 2016; 139:617–27.

13. Glattre E, Haldorsen T, Berg JP, Stensvold I, Solvoll K. Norwegian case-control study testing the hypothesis that seafood increases the risk of thyroid cancer. Cancer Causes Control. 1993; 4:11–6.

14. Wingren G, Hatschek T, Axelson O. Determinants of papillary cancer of the thyroid. Am J Epidemiol. 1993; 138:482–91.

15. Preston-Martin S, Bernstein L, Pike MC, Maldonado AA, Henderson BE. Thyroid cancer among young women related to prior thyroid disease and pregnancy history. Br J Cancer. 1987; 55:191–5.

16. D’Avanzo B, La Vecchia C, Franceschi S, Negri E, Talamini R. History of thyroid diseases and subsequent thyroid cancer risk. Cancer Epidemiol Biomarkers Prev. 1995; 4:193–9.
17. Xhaard C, Ren Y, Clero E, Maillard S, Brindel P, Rachedi F, et al. Differentiated thyroid carcinoma risk factors in French Polynesia. Asian Pac J Cancer Prev. 2014; 15:2675–80.

18. Hallquist A, Hardell L, Degerman A, Boquist L. Thyroid cancer: reproductive factors, previous diseases, drug intake, family history and diet. A case-control study. Eur J Cancer Prev. 1994; 3:481–8.
19. Blakely T, Barendregt JJ, Foster RH, Hill S, Atkinson J, Sarfati D, et al. The association of active smoking with multiple cancers: national census-cancer registry cohorts with quantitative bias analysis. Cancer Causes Control. 2013; 24:1243–55.

20. Sawicka-Gutaj N, Gutaj P, Sowinski J, Wender-Ozegowska E, Czarnywojtek A, Brazert J, et al. Influence of cigarette smoking on thyroid gland: an update. Endokrynol Pol. 2014; 65:54–62.
21. Wiersinga WM. Smoking and thyroid. Clin Endocrinol (Oxf). 2013; 79:145–51.

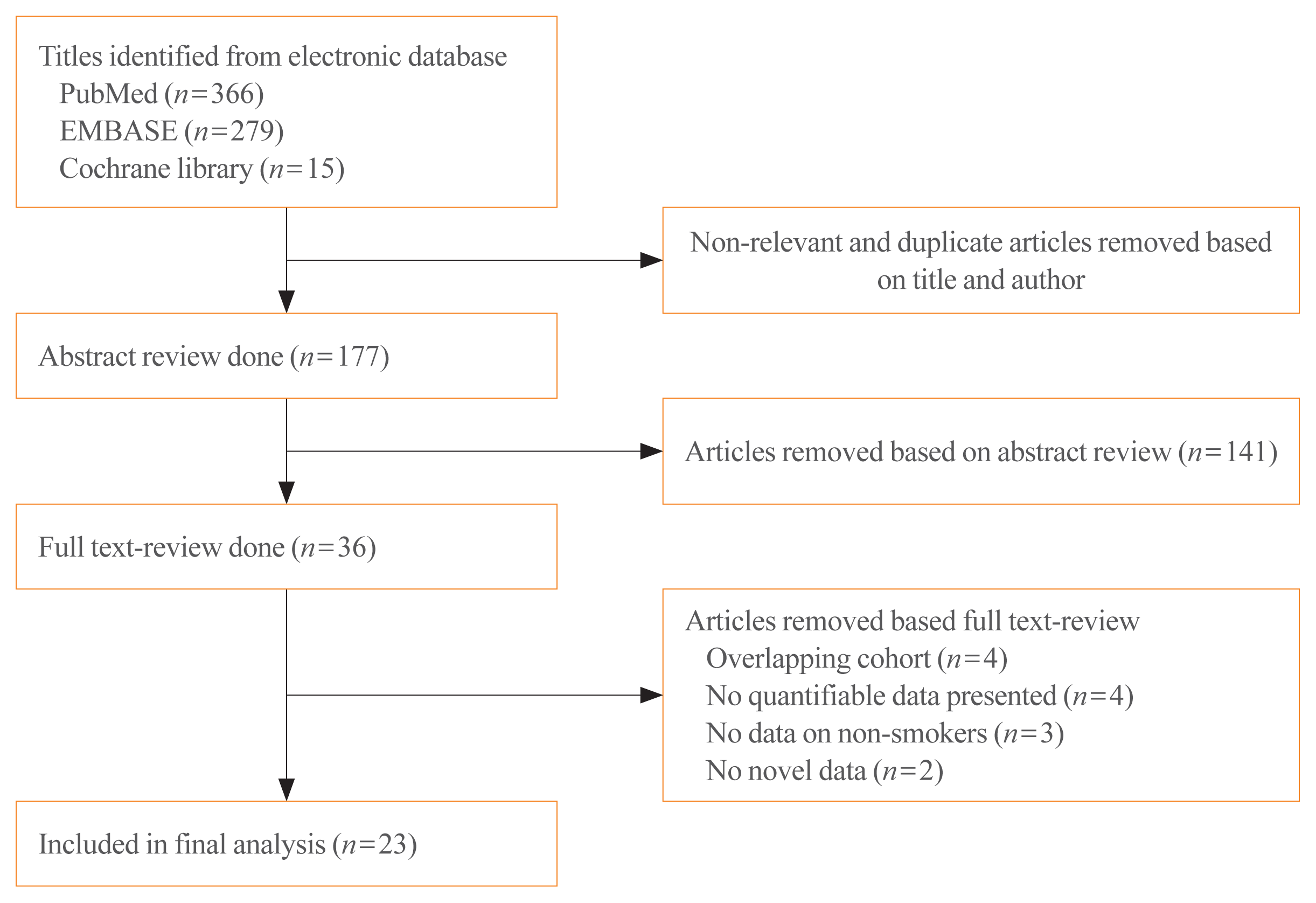
22. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010; 8:336–41.

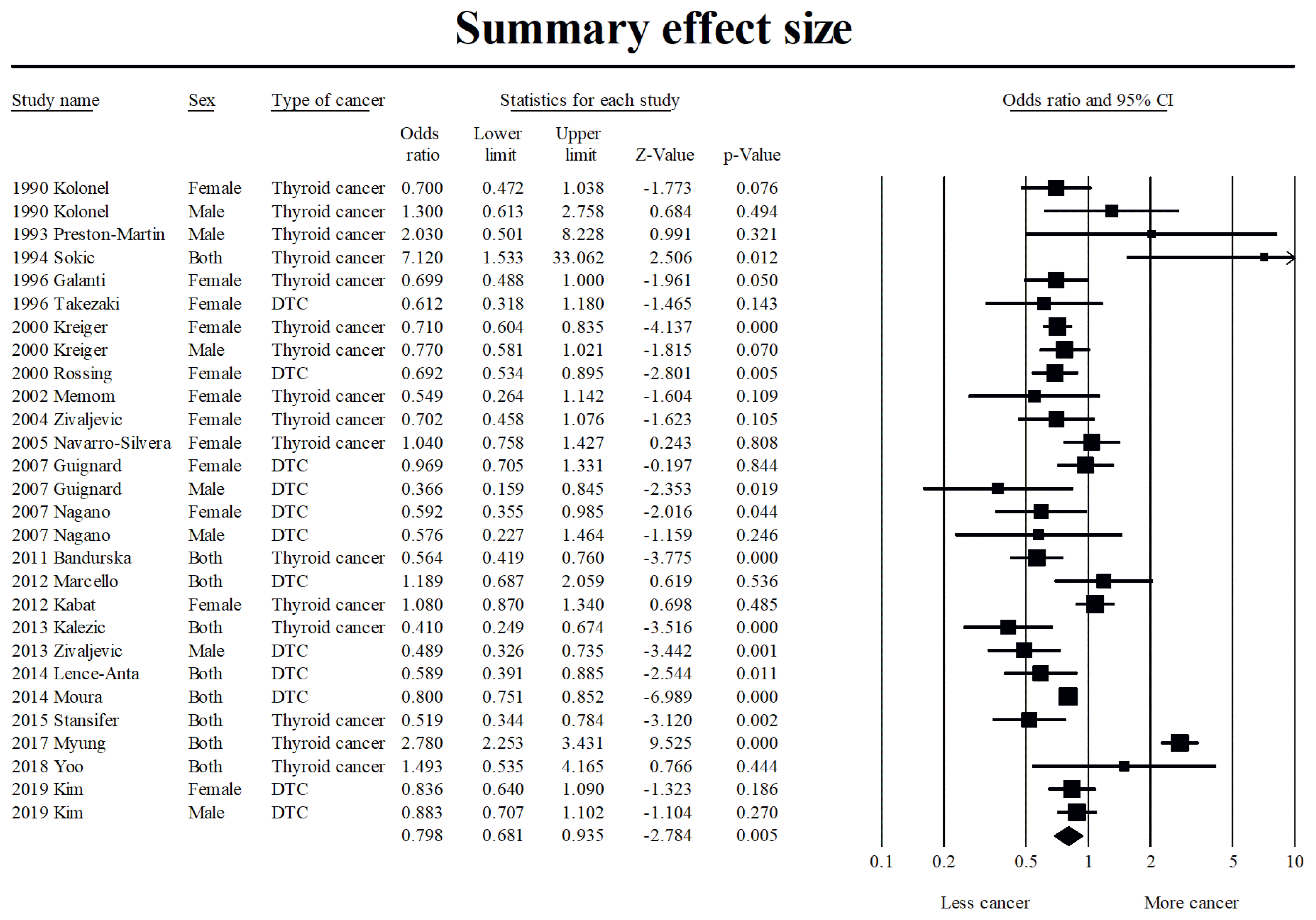
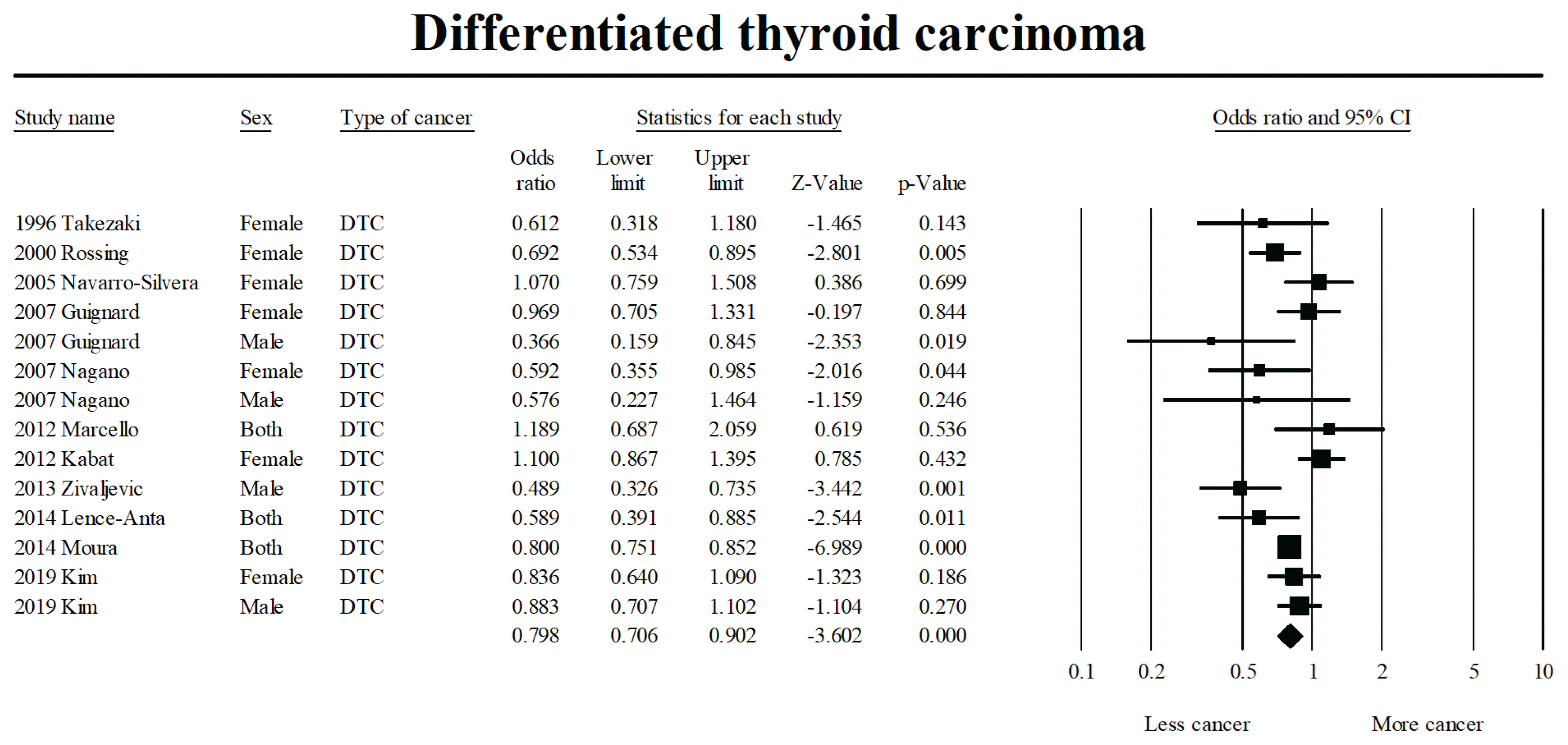
23. DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015; 45(Pt A):139–45.

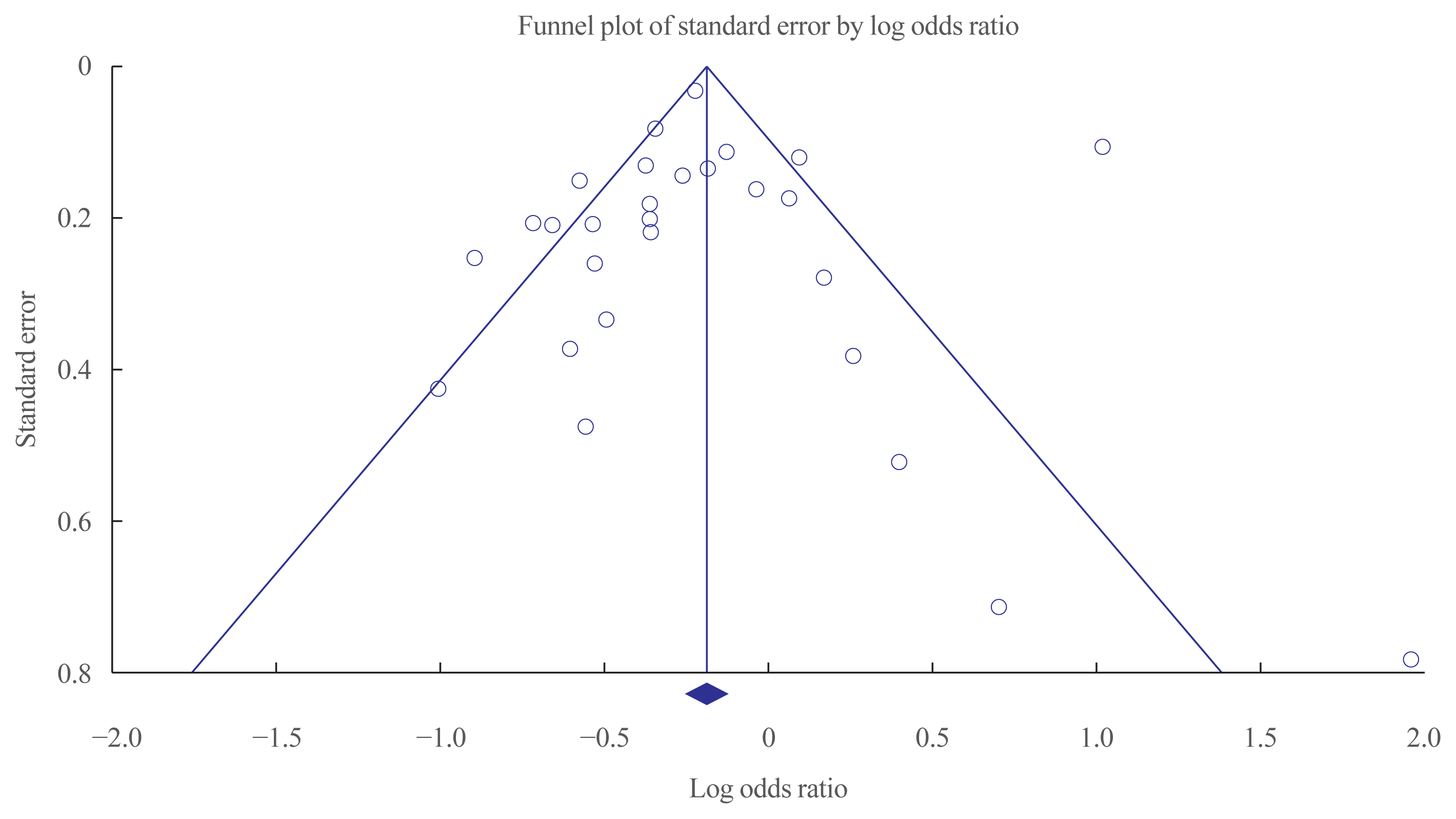
24. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997; 315:629–34.

25. Wells G, Shea B, O’Connell D. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomized studies in meta-analysis. In : Proceedings of the Third Symposium on Systematic Reviews beyond the Basics: Improving Quality and Impact; 2000 Jul 4–9; Oxford, UK. Oxford: Oxford University Press;2000.
26. Kolonel LN, Hankin JH, Wilkens LR, Fukunaga FH, Hinds MW. An epidemiologic study of thyroid cancer in Hawaii. Cancer Causes Control. 1990; 1:223–34.

27. Preston-Martin S, Jin F, Duda MJ, Mack WJ. A case-control study of thyroid cancer in women under age 55 in Shanghai (People’s Republic of China). Cancer Causes Control. 1993; 4:431–40.

28. Galanti MR, Hansson L, Lund E, Bergstrom R, Grimelius L, Stalsberg H, et al. Reproductive history and cigarette smoking as risk factors for thyroid cancer in women: a population-based case-control study. Cancer Epidemiol Biomarkers Prev. 1996; 5:425–31.
29. Takezaki T, Hirose K, Inoue M, Hamajima N, Kuroishi T, Nakamura S, et al. Risk factors of thyroid cancer among women in Tokai, Japan. J Epidemiol. 1996; 6:140–7.

30. Kreiger N, Parkes R. Cigarette smoking and the risk of thyroid cancer. Eur J Cancer. 2000; 36:1969–73.

31. Rossing MA, Cushing KL, Voigt LF, Wicklund KG, Daling JR. Risk of papillary thyroid cancer in women in relation to smoking and alcohol consumption. Epidemiology. 2000; 11:49–54.

32. Memon A, Darif M, Al-Saleh K, Suresh A. Epidemiology of reproductive and hormonal factors in thyroid cancer: evidence from a case-control study in the Middle East. Int J Cancer. 2002; 97:82–9.

33. Zivaljevic V, Vlajinac H, Marinkovic J, Paunovic I, Diklic A, Dzodic R. Cigarette smoking as a risk factor for cancer of the thyroid in women. Tumori. 2004; 90:273–5.

34. Nagano J, Mabuchi K, Yoshimoto Y, Hayashi Y, Tsuda N, Land C, et al. A case-control study in Hiroshima and Nagasaki examining non-radiation risk factors for thyroid cancer. J Epidemiol. 2007; 17:76–85.

35. Bandurska-Stankiewicz E, Aksamit-Bialoszewska E, Rutkowska J, Stankiewicz A, Shafie D. The effect of nutritional habits and addictions on the incidence of thyroid carcinoma in the Olsztyn province of Poland. Endokrynol Pol. 2011; 62:145–50.
36. Marcello MA, Sampaio AC, Geloneze B, Vasques AC, Assumpcao LV, Ward LS. Obesity and excess protein and carbohydrate consumption are risk factors for thyroid cancer. Nutr Cancer. 2012; 64:1190–5.

37. Kalezic NK, Zivaljevic VR, Slijepcevic NA, Paunovic IR, Diklic AD, Sipetic SB. Risk factors for sporadic medullary thyroid carcinoma. Eur J Cancer Prev. 2013; 22:262–7.

38. Zivaljevic V, Slijepcevic N, Sipetic S, Paunovic I, Diklic A, Zoric G, et al. Risk factors for well-differentiated thyroid cancer in men. Tumori. 2013; 99:458–62.

39. Lence-Anta JJ, Xhaard C, Ortiz RM, Kassim H, Pereda CM, Turcios S, et al. Environmental, lifestyle, and anthropometric risk factors for differentiated thyroid cancer in cuba: a case-control study. Eur Thyroid J. 2014; 3:189–96.

40. Moura MA, Bergmann A, Aguiar SS, Thuler LC. The magnitude of the association between smoking and the risk of developing cancer in Brazil: a multicenter study. BMJ Open. 2014; 4:e003736.

41. Stansifer KJ, Guynan JF, Wachal BM, Smith RB. Modifiable risk factors and thyroid cancer. Otolaryngol Head Neck Surg. 2015; 152:432–7.

42. Myung SK, Lee CW, Lee J, Kim J, Kim HS. Risk factors for thyroid cancer: a hospital-based case-control study in Korean adults. Cancer Res Treat. 2017; 49:70–8.

43. Yoo YG, Yu BJ, Choi EH. A comparison study: the risk factors in the lifestyles of thyroid cancer patients and healthy adults of South Korea. Cancer Nurs. 2018; 41:E48–56.
44. Kim KN, Hwang Y, Kim K, Lee KE, Park YJ, Choi JY, et al. Active and passive smoking, BRAFV600E mutation status, and the risk of papillary thyroid cancer: a large-scale case-control and case-only study. Cancer Res Treat. 2019; 51:1392–9.

45. Navarro Silvera SA, Miller AB, Rohan TE. Risk factors for thyroid cancer: a prospective cohort study. Int J Cancer. 2005; 116:433–8.

46. Kabat GC, Kim MY, Wactawski-Wende J, Rohan TE. Smoking and alcohol consumption in relation to risk of thyroid cancer in postmenopausal women. Cancer Epidemiol. 2012; 36:335–40.

47. Cho YA, Kim J. Thyroid cancer risk and smoking status: a meta-analysis. Cancer Causes Control. 2014; 25:1187–95.

48. Boelaert K, Horacek J, Holder RL, Watkinson JC, Sheppard MC, Franklyn JA. Serum thyrotropin concentration as a novel predictor of malignancy in thyroid nodules investigated by fine-needle aspiration. J Clin Endocrinol Metab. 2006; 91:4295–301.

49. Hayashi M, Futawaka K, Matsushita M, Hatai M, Yoshikawa N, Nakamura K, et al. Cigarette smoke extract disrupts transcriptional activities mediated by thyroid hormones and its receptors. Biol Pharm Bull. 2018; 41:383–93.

50. Czarnywojtek A, Warmuz-Stangierska I, Zdanowska J, Florek E, Zgorzlewicz M, Ruchala M, et al. Smoking and thyroid disease: review of literature. Przegl Lek. 2009; 66:878–81.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download