1. Bilezikian JP, Khan AA, Potts JT Jr. Third International Workshop on the Management of Asymptomatic Primary Hyperthyroidism. Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the third international workshop. J Clin Endocrinol Metab. 2009; 94:335–9.
2. Silverberg SJ, Clarke BL, Peacock M, Bandeira F, Boutroy S, Cusano NE, et al. Current issues in the presentation of asymptomatic primary hyperparathyroidism: proceedings of the Fourth International Workshop. J Clin Endocrinol Metab. 2014; 99:3580–94.

3. Cusano NE, Silverberg SJ, Bilezikian JP. Normocalcemic primary hyperparathyroidism. J Clin Densitom. 2013; 16:33–9.

4. Coe FL, Canterbury JM, Firpo JJ, Reiss E. Evidence for secondary hyperparathyroidism in idiopathic hypercalciuria. J Clin Invest. 1973; 52:134–42.

5. Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011; 96:53–8.

6. Fuleihan Gel-H, Bouillon R, Clarke B, Chakhtoura M, Cooper C, McClung M, et al. Serum 25-hydroxyvitamin D levels: variability, knowledge gaps, and the concept of a desirable range. J Bone Miner Res. 2015; 30:1119–33.

7. Eastell R, Brandi ML, Costa AG, D’Amour P, Shoback DM, Thakker RV. Diagnosis of asymptomatic primary hyperparathyroidism: proceedings of the Fourth International Workshop. J Clin Endocrinol Metab. 2014; 99:3570–9.

8. Giustina A, Adler RA, Binkley N, Bouillon R, Ebeling PR, Lazaretti-Castro M, et al. Controversies in vitamin D: summary statement from an international conference. J Clin Endocrinol Metab. 2019; 104:234–40.

9. Madeo B, De Vincentis S, Repaci A, Altieri P, Vicennati V, Kara E, et al. The calcium-to-phosphorous (Ca/P) ratio in the diagnosis of primary hyperparathyroidism and hypoparathyroidism: a multicentric study. Endocrine. 2020; 68:679–87.

10. Schini M, Jacques RM, Oakes E, Peel NFA, Walsh JS, Eastell R. Normocalcemic hyperparathyroidism: study of its prevalence and natural history. J Clin Endocrinol Metab. 2020; 105:e1171–86.

11. Zaidan J, Wang X. Letter to the editor: “Normocalcemic hyperparathyroidism: study of its prevalence and natural history”. J Clin Endocrinol Metab. 2020; 105:dgaa275.

12. Schini M, Eastell R. Response to letter to the editor: “Normocalcemic hyperparathyroidism: study of its prevalence and natural history”. J Clin Endocrinol Metab. 2020; 105:e2689.

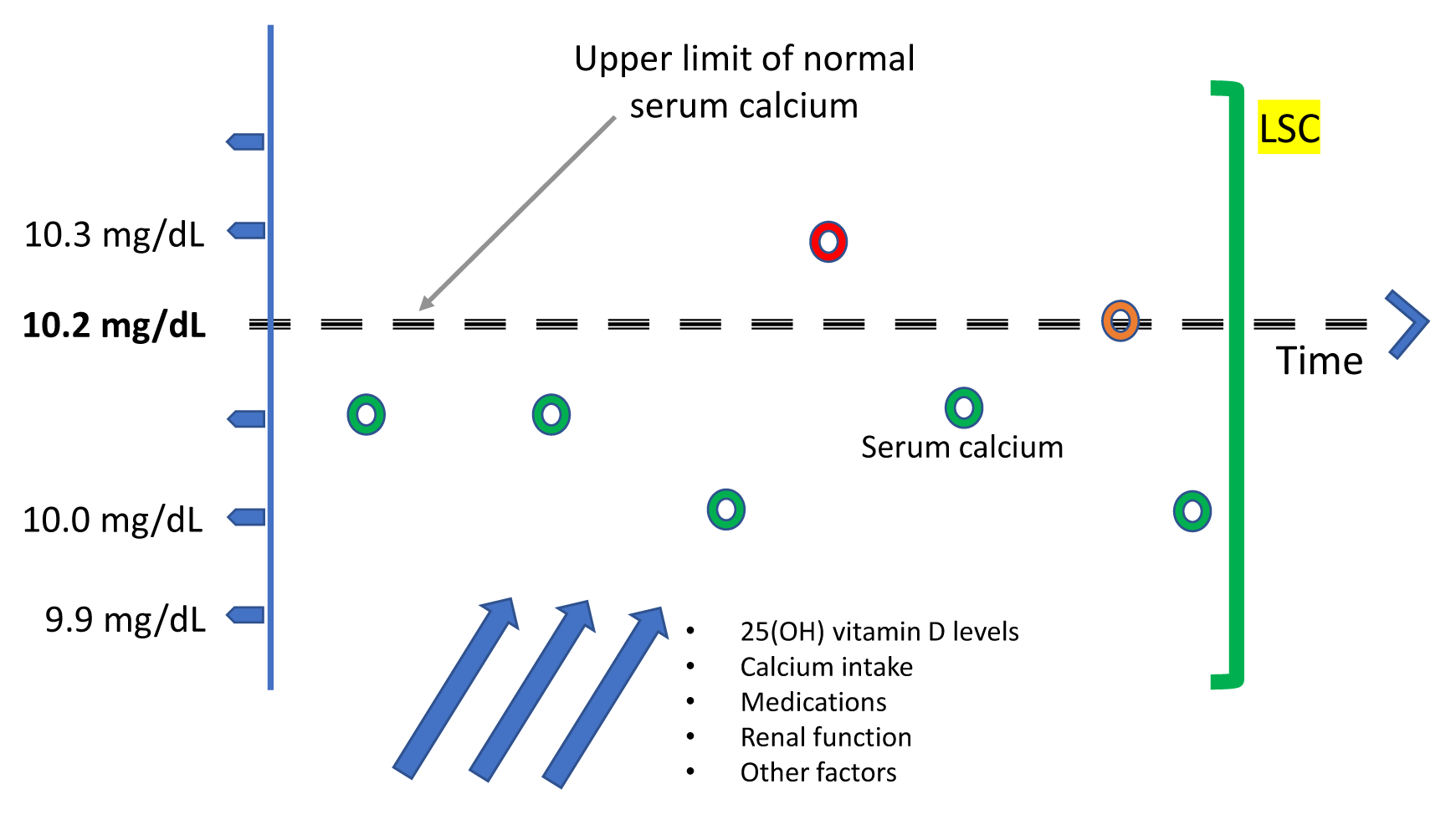
13. Schini M, Jacques R, Oakes E, Peel N, Walsh JS, Eastell R. Normocalcaemic hyperparathyroidism and primary hyperparathyroidism: least significant change for adjusted serum calcium. Eur J Endocrinol. 2021; 184:K7–10.

14. Bland JM, Altman DG. Measurement error. BMJ. 1996; 313:744.
15. Cusano NE, Maalouf NM, Wang PY, Zhang C, Cremers SC, Haney EM, et al. Normocalcemic hyperparathyroidism and hypoparathyroidism in two community-based nonreferral populations. J Clin Endocrinol Metab. 2013; 98:2734–41.

16. Lundgren E, Rastad J, Thrufjell E, Akerstrom G, Ljunghall S. Population-based screening for primary hyperparathyroidism with serum calcium and parathyroid hormone values in menopausal women. Surgery. 1997; 121:287–94.

17. Berger C, Almohareb O, Langsetmo L, Hanley DA, Kovacs CS, Josse RG, et al. Characteristics of hyperparathyroid states in the Canadian multicentre osteoporosis study (CaMos) and relationship to skeletal markers. Clin Endocrinol (Oxf). 2015; 82:359–68.

18. Kontogeorgos G, Trimpou P, Laine CM, Olerod G, Lindahl A, Landin-Wilhelmsen K. Normocalcaemic, vitamin D-sufficient hyperparathyroidism: high prevalence and low morbidity in the general population. A long-term follow-up study, the WHO MONICA project, Gothenburg, Sweden. Clin Endocrinol (Oxf). 2015; 83:277–84.
19. Garcia-Martin A, Reyes-Garcia R, Munoz-Torres M. Normocalcemic primary hyperparathyroidism: one-year follow-up in one hundred postmenopausal women. Endocrine. 2012; 42:764–6.

20. Amaral LM, Queiroz DC, Marques TF, Mendes M, Bandeira F. Normocalcemic versus hypercalcemic primary hyperparathyroidism: more stone than bone? J Osteoporos. 2012; 2012:128352.

21. Maruani G, Hertig A, Paillard M, Houillier P. Normocalcemic primary hyperparathyroidism: evidence for a generalized target-tissue resistance to parathyroid hormone. J Clin Endocrinol Metab. 2003; 88:4641–8.

22. Tordjman KM, Greenman Y, Osher E, Shenkerman G, Stern N. Characterization of normocalcemic primary hyperparathyroidism. Am J Med. 2004; 117:861–3.

23. Silverberg SJ, Bilezikian JP. “Incipient” primary hyperparathyroidism: a “forme fruste” of an old disease”. J Clin Endocrinol Metab. 2003; 88:5348–52.

24. Lowe H, McMahon DJ, Rubin MR, Bilezikian JP, Silverberg SJ. Normocalcemic primary hyperparathyroidism: further characterization of a new clinical phenotype. J Clin Endocrinol Metab. 2007; 92:3001–5.

25. Wade TJ, Yen TW, Amin AL, Wang TS. Surgical management of normocalcemic primary hyperparathyroidism. World J Surg. 2012; 36:761–6.

26. Koumakis E, Souberbielle JC, Sarfati E, Meunier M, Maury E, Gallimard E, et al. Bone mineral density evolution after successful parathyroidectomy in patients with normocalcemic primary hyperparathyroidism. J Clin Endocrinol Metab. 2013; 98:3213–20.

27. Marques TF, Vasconcelos R, Diniz E, Rego D, Griz L, Bandeira F. Normocalcemic primary hyperparathyroidism in clinical practice: an indolent condition or a silent threat? Arq Bras Endocrinol Metabol. 2011; 55:314–7.

28. Cakir I, Unluhizarci K, Tanriverdi F, Elbuken G, Karaca Z, Kelestimur F. Investigation of insulin resistance in patients with normocalcemic primary hyperparathyroidism. Endocrine. 2012; 42:419–22.

29. Siprova H, Frysak Z, Soucek M. Primary hyperparathyroidism, with a focus on management of the normocalcemic form: to treat or not to treat? Endocr Pract. 2016; 22:294–301.

30. Palermo A, Naciu AM, Tabacco G, Falcone S, Santonati A, Maggi D, et al. Clinical, biochemical, and radiological profile of normocalcemic primary hyperparathyroidism. J Clin Endocrinol Metab. 2020; 105:dgaa174.

31. Lemos ALP, Andrade SRL, Pontes LLH, Teixeira PMC, Bandeira E, Bandeira LC, et al. High rate of occult urolithiasis in normocalcemic primary hyperparathyroidism. Kidney Blood Press Res. 2019; 44:1189–95.

32. Silverberg SJ, Shane E, Jacobs TP, Siris E, Bilezikian JP. A 10-year prospective study of primary hyperparathyroidism with or without parathyroid surgery. N Engl J Med. 1999; 341:1249–55.

33. Cesareo R, Di Stasio E, Vescini F, Campagna G, Cianni R, Pasqualini V, et al. Effects of alendronate and vitamin D in patients with normocalcemic primary hyperparathyroidism. Osteoporos Int. 2015; 26:1295–302.

34. Tang J, Mettler P, McFann K, Chonchol M. The association of prevalent kidney stone disease with mortality in US adults: the National Health and Nutrition Examination Survey III, 1988–1994. Am J Nephrol. 2013; 37:501–6.

35. Brardi S, Cevenini G, Verdacchi T, Romano G, Ponchietti R. Use of cinacalcet in nephrolithiasis associated with normocalcemic or hypercalcemic primary hyperparathyroidism: results of a prospective randomized pilot study. Arch Ital Urol Androl. 2015; 87:66–71.

36. Saxena T, Ali AO, Saxena M. Pathophysiology of essential hypertension: an update. Expert Rev Cardiovasc Ther. 2018; 16:879–87.

37. Rossi GP, Lenzini L. Vitamin D supplementation: a novel therapy for aldosteronism? Nat Rev Endocrinol. 2020; 16:303–4.

38. Tomaschitz A, Ritz E, Pieske B, Rus-Machan J, Kienreich K, Verheyen N, et al. Aldosterone and parathyroid hormone interactions as mediators of metabolic and cardiovascular disease. Metabolism. 2014; 63:20–31.

39. Oinonen L, Tikkakoski A, Koskela J, Eraranta A, Kahonen M, Niemela O, et al. Parathyroid hormone may play a role in the pathophysiology of primary hypertension. Endocr Connect. 2021; 10:54–65.

40. Kometani M, Yoneda T, Aono D, Gondoh-Noda Y, Matsuoka T, Higashitani T, et al. Primary aldosteronism with parathyroid hormone elevation: a single-center retrospective study. Intern Med. 2021; 60:993–8.

41. Chen G, Xue Y, Zhang Q, Xue T, Yao J, Huang H, et al. Is normocalcemic primary hyperparathyroidism harmful or harmless? J Clin Endocrinol Metab. 2015; 100:2420–4.

42. Beysel S, Caliskan M, Kizilgul M, Apaydin M, Kan S, Ozbek M, et al. Parathyroidectomy improves cardiovascular risk factors in normocalcemic and hypercalcemic primary hyperparathyroidism. BMC Cardiovasc Disord. 2019; 19:106.

43. Mesquita PN, Dornelas Leao Leite AP, Chagas Crisostomo SD, Veras Filho E, da Cunha Xavier L, Bandeira F. Evaluation of coronary calcium score in patients with normocalcemic primary hyperparathyroidism. Vasc Health Risk Manag. 2017; 13:225–9.

44. Pepe J, Colangelo L, Sonato C, Occhiuto M, Ferrara C, Del Fattore A, et al. Echocardiographic findings in patients with normocalcemic primary hyperparathyroidism compared with findings in hypercalcemic primary hyperparathyroid patients and control subjects. Endocr Pract. 2021; 27:21–6.
45. Bollerslev J, Sjostedt E, Rejnmark L. Cardiovascular consequences of parathyroid disorders in adults. Ann Endocrinol (Paris). 2021; 82:151–7.

46. Karras SN, Koufakis T, Tsekmekidou X, Antonopoulou V, Zebekakis P, Kotsa K. Increased parathyroid hormone is associated with higher fasting glucose in individuals with normocalcemic primary hyperparathyroidism and prediabetes: a pilot study. Diabetes Res Clin Pract. 2020; 160:107985.

47. Karras S, Annweiler C, Kiortsis D, Koutelidakis I, Kotsa K. Improving glucose homeostasis after parathyroidectomy for normocalcemic primary hyperparathyroidism with co-existing prediabetes. Nutrients. 2020; 12:3522.

48. Pacifici R. Role of gut microbiota in the skeletal response to PTH. J Clin Endocrinol Metab. 2021; 106:636–45.

49. Bannani S, Christou N, Guerin C, Hamy A, Sebag F, Mathonnet M, et al. Effect of parathyroidectomy on quality of life and non-specific symptoms in normocalcaemic primary hyperparathyroidism. Br J Surg. 2018; 105:223–9.

50. Voss L, Nobrega M, Bandeira L, Griz L, Rocha-Filho PAS, Bandeira F. Impaired physical function and evaluation of quality of life in normocalcemic and hypercalcemic primary hyperparathyroidism. Bone. 2020; 141:115583.

51. Pretorius M, Lundstam K, Hellstrom M, Fagerland MW, Godang K, Mollerup C, et al. Effects of parathyroidectomy on quality of life: 10 years of data from a prospective randomized controlled trial on primary hyperparathyroidism (the SIPH-Study). J Bone Miner Res. 2021; 36:3–11.

52. Pandian TK, Lubitz CC, Bird SH, Kuo LE, Stephen AE. Normocalcemic hyperparathyroidism: a Collaborative Endocrine Surgery Quality Improvement Program analysis. Surgery. 2020; 167:168–72.

53. Trinh G, Rettig E, Noureldine SI, Russell JO, Agrawal N, Mathur A, et al. Surgical management of normocalcemic primary hyperparathyroidism and the impact of intraoperative parathyroid hormone testing on outcome. Otolaryngol Head Neck Surg. 2018; 159:630–7.

54. Graves CE, McManus CM, Chabot JA, Lee JA, Kuo JH. Biochemical profile affects IOPTH kinetics and cure rate in primary hyperparathyroidism. World J Surg. 2020; 44:488–95.

55. Sho S, Kuo EJ, Chen AC, Li N, Yeh MW, Livhits MJ. Biochemical and skeletal outcomes of parathyroidectomy for normocalcemic (incipient) primary hyperparathyroidism. Ann Surg Oncol. 2019; 26:539–46.

56. Traini E, Bellantone R, Tempera SE, Russo S, De Crea C, Lombardi CP, et al. Is parathyroidectomy safe and effective in patients with normocalcemic primary hyperparathyroidism? Langenbecks Arch Surg. 2018; 403:317–23.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download