Abstract
Background
Concerns have been raised regarding thyroid disorders caused by excessive iodine in Koreans, who have iodine-rich diets. This study evaluated iodine status using dietary iodine intake and urinary iodine in papillary thyroid cancer (PTC) patients.
Methods
Dietary data of PTC patients were assessed using a 24-hour recall and food frequency questionnaire (FFQ), and urinary iodine concentrations (UICs) were also obtained. To compare the iodine status of PTC patients, Korean adults with or without thyroid disease from the Korea National Health and Nutrition Examination Survey, which had 24-hour recall data and urinary iodine measurements, were analyzed.
Results
The median daily iodine intake by 24-hour recall was 341.7 μg/day in PTC patients, similar to the levels of other Korean adults. Based on UICs, the prevalence of excessive iodine was 54.4% in PTC patients, which was similar to the prevalence among subjects with thyroid disease (55.4%) but slightly higher than that in subjects without thyroid disease (47.7%). Based on dietary iodine by 24-hour recall, the prevalence of excessive iodine intake was 7.2%, which was higher than that among subjects with (4.4%) or without (3.9%) thyroid disease. The dietary iodine intake based on 24-hour recall was closely correlated with the UIC (r=0.4826) in PTC patients, but dietary iodine by FFQ was not significantly correlated with either 24-hour recall or UIC-based dietary iodine.
Adequate intake of iodine is essential for thyroid hormone synthesis and function. Increasing evidence from epidemiological studies has demonstrated a U-shaped relationship between iodine intake and thyroid disease, including thyroid cancer [1]. A study of Danish women showed a high prevalence of goiter in an area where iodine intake was moderately low [2], and a study of a Chinese cohort from three rural areas showed a higher prevalence of goiter in a region with mild iodine deficiency than in other regions with more than adequate iodine intake [3]. In contrast, a meta-analysis of 16 case-control studies showed a positive association between high iodine levels and papillary thyroid cancer (PTC) [4].
Korea is an iodine-replete area in which foods from the sea, particularly certain types of seaweed with high iodine content, are commonly consumed in a typical diet. As most ingested iodine is excreted within 48 hours, the urinary iodine concentration (UIC) serves as a biomarker for recent dietary iodine intake. The median UIC in Koreans who participated in the Korea National Health and Nutrition Examination Survey (KNHANES) was 293.9 μg/L, which is above the requirement according to the World Health Organization (WHO) iodine recommendations [5]. A study of Korean preschool children showed that the median UIC was 438.8 μg/L, and 66.4% of subjects exhibited excessive iodine intake (300 μg/L) [6].
Although the UIC is a well-accepted, cost-effective, and readily available indicator for evaluating the iodine nutritional status of a population [7], it is necessary to estimate dietary iodine intake at the population level, identify major food sources, and establish dietary guidelines for proper iodine intake in order to minimize the risk of thyroid cancer or thyroid disorders in Koreans whose habitual diets involve high iodine intake.
Due to the lack of an iodine database, few studies have reported dietary iodine intake across countries. The difficulty in establishing an iodine database lies in the nature and degree of variability in foods. Carriquiry et al. [8] reported temporal and regional variability in eight foods over time using data from the Total Diet Study in areas of the United States where measured iodine concentrations of foods were available. They recommended that food composition tables provide useful information on variability, including median levels for nutrients such as iodine with highly variable concentrations in major food sources. As there is no national iodine database for Japan, Katagiri et al. [9] developed an iodine database based on imputation and the food composition tables of other countries. Choi et al. [10] recently revised the iodine database for 855 Korean foods with updated references, including the iodine content of types of seaweed released by the Korea Ministry of Food and Drugs. A study of Flemish preschool children reported variation in dietary iodine after applying German or British food composition tables due to the absence of an iodine database in Belgium [11].
The relationship between dietary iodine intake and thyroid function is complex. A meta-analysis of eight case-control studies showed that higher iodine intake and high consumption of saltwater fish and shellfish were protective factors against thyroid cancer [12]. However, another meta-analysis showed that a high total amount of fish in an iodine-non-deficient area was associated with a slightly increased risk of thyroid cancer. The authors concluded that dietary factors can differ based on iodine availability [13]. This suggests that dietary iodine and its relationship with thyroid disease should be explored according to iodine availability.
Although data on dietary iodine intake are limited, the dietary iodine levels of Korean and Japanese participants were reported to be notably higher than those in European countries. The median iodine intake in Japanese adults based on 28-day dietary records was 312 μg/day in men and 413 μg/day in women [14], and that in Korean adults was 400.8 μg/day in men and 311.2 μg/day in women based on a single 24-hour recall [10]. In contrast, the median iodine intake in pregnant UK women was reportedly 190 μg/day using a food frequency questionnaire (FFQ) [15], and the average iodine intake in French women based on a diet-history questionnaire was 155.6 μg/day [16]. However, it is difficult to compare these values directly due to the different methods used to assess dietary intake.
As certain foods have high iodine content, 24-hour recall or dietary-record methods for measuring dietary intake need to examine multiple days of intake, and an FFQ is needed to validate iodine-specific items and estimate usual iodine intake. Combet and Lean [17] developed a short FFQ specifically for measuring iodine intake in UK women, but its 17 items included no seaweed food items, which are commonly consumed in Korea and Japan. Therefore, to elucidate the role of dietary iodine in thyroid cancer or other disorders caused by excessive iodine intake, more studies focusing on Koreans and their iodine-rich diet are necessary.
Thus, the aim of this study was to evaluate iodine status using dietary iodine intake measured with 24-hour recall and an FFQ, as well as UICs of patients with PTC from a tertiary hospital. As a comparison group for the PTC patients, Korean adults from the KNHANES who had the same dietary and urinary iodine measurements were analyzed.
Subjects with PTC were recruited from Gachon University Gil Medical Center. From October 2017 through February 2019, a total of 328 patients who were scheduled to undergo thyroidectomy agreed to participate in this study. Among them, subjects who completed a 24-hour food recall (n=223) an FFQ (n=294), had urinary iodine data (n=274), and had all measurements (n=204) were included for further analyses (Fig. 1). The study protocol was approved by the Institutional Review Board of Gachon University Gil Medical Center (GCIRB2017-303). The study complied with the Declaration of Helsinki in its latest version.
Data for subjects with or without thyroid disease were obtained from the 2013 to 2015 KNHANES to compare dietary and urinary iodine levels with those in patients with PTC. The KNHANES is a nationwide survey that consists of a health interview, health examination, and dietary survey, and the 2013 to 2015 survey, in particular, included urinary iodine in the health examination. Detailed information regarding the survey procedures has been provided elsewhere [18]. Among 15,860 adults aged 20 years or older who participated in a 24-hour recall, those who had no urinary iodine measurements (n=9,619), were missing body mass index or blood data (n=139), were pregnant or lactating (n=84), or reported implausible energy intake in a 24-hour period (n=91) were excluded. Of the total of 5,927 subjects, those who had been diagnosed with thyroid disease, excluding thyroid cancer (n=47), by a doctor were grouped together as subjects with thyroid disease (n=184); the others were grouped as subjects without thyroid disease (n=5,696). The study protocol was approved by the Institutional Review Board of the Korea Centers for Disease Control and Prevention (2013-07CON-03-4C, 2013-12EXP-03-5C, 2015-01-02-6C). All participants provided written informed consent.
Dietary assessments were conducted in the hospital using two methods: 24-hour recall and an FFQ. The 24-hour recall was administered by a trained interviewer on the day before surgery. Subjects were asked for detailed information on all foods and beverages consumed before coming to the hospital, as they were scheduled to fast for surgery after admission and to collect urine the next morning. As most ingested iodine is excreted in urine within 24 hours, subjects completed the 24-hour recall on the day of admission to the hospital. An FFQ, which was previously validated for Korean adults and had been used for the Korean Genome Epidemiology Study [19], was also administered by a trained interviewer. Each subject was asked to provide their usual consumption of 106 food items over the past 12 months.
Dietary data for KNHANES were obtained through a single-day 24-hour recall administered at each participant’s household during which the participant was asked for detailed information on all food and beverages consumed in the past 24 hours.
Dietary data from the hospital and KNHANES were linked to an official food composition table established by the Rural Development Administration of Korea, and then energy and nutrient intake from foods and beverages were determined for each subject. However, the recent ninth Korean Food Composition Table has incomplete information on iodine, so the newly revised iodine database for common Korean foods was used in this study to calculate iodine intake [10]. Briefly, iodine content in common Korean foods was matched by the food code in dietary data obtained by 24-hour recall or an FFQ, and then each participant’s daily iodine intake was calculated.
Dietary iodine was evaluated using 2020 dietary reference intakes (DRIs) for Koreans [20]. For iodine, the estimated average requirement (EAR) is 95 μg/day, the recommended nutrient intake (RNI) is 150 μg/day, and the tolerable upper intake level (UL) is 2,400 μg/day for adults aged 19 years or older. According to the DRIs for iodine, dietary iodine was classified as follows: dietary iodine below the EAR (<95 μg/day) was considered deficient, and dietary iodine above the UL (>2,400 μg/day) was considered excessive.
To measure UIC, a single, fasting, spot urine sample was collected in the hospital on the morning of surgery. Urine samples were frozen at −20°C and sent to a certified laboratory for analysis. The UIC was then analyzed via inductively coupled plasma mass spectrometry (ICP-MS) using an Agilent 7500 series instrument (Agilent Technologies Inc., Tokyo, Japan). The UIC data from the KNHANES were obtained from single spot urine specimens analyzed using ICP-MS, as previously described [5].
The UIC was evaluated according to the WHO iodine recommendations [21], according to which urinary iodine was determined as insufficient (UIC <100 μg/L), adequate (UIC 100 to 199 μg/L), more than adequate (UIC 200 to 299 μg/L), or excessive (UIC ≥300 μg/L).
To examine the agreement and correlation of UIC with dietary iodine, the ratio of urinary iodine to creatinine (I/Cr) was used to represent urine iodine, as the I/Cr ratio from spot urine samples could serve as a useful and reliable alternative to 24-hour urine collection.
All statistical analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). All continuous variables were presented as the mean±standard deviation, and all categorical variables related to basic characteristics and nutrient intake were presented as numbers with percentages (%). As dietary iodine was highly skewed, the medians and interquartile ranges (25th and 75th percentiles) were presented along with the means and 90th percentiles in a box-and-whisker plot. The contributions of various food groups to total iodine intake according to the 24-hour recall were calculated as the percentage of iodine consumed in each food group relative to the total iodine intake of all subjects. The classification of food groups was based on the ninth Korean Food Composition Table; the major food groups that contribute to >0.5% of the total iodine intake are presented. UIC data were presented according to the proportion of subjects in various groups defined by WHO recommendations; further categories (e.g., 300–499, 500–999, 1,000–1,999, 2,000–2,999, and ≥3,000 μg/L) were applied to analyze the distribution of subjects with excessive iodine.
The level of agreement was calculated between the two measurement methods. Each set of measurements was divided into quintiles, and agreement was defined as the percentage of both measurements that matched within ±1 quintile or ±2 quintiles. Correlation analysis was also used to determine associations between the two measurement methods using Pearson and Spearman correlation coefficients after adjustment for age, sex, and energy intake, in which all measurements were log-transformed due to the highly skewed nature of the data, and the urinary I/Cr ratio was used to represent urinary iodine. P values <0.05 were considered to indicate statistical significance.
The basic characteristics and overall nutrient intake of hospital patients with PTC and KNHANES subjects with or without thyroid disease are summarized in Table 1, which presents a comparison of dietary iodine according to thyroid disease status. The mean age was 48.9 years in patients with thyroid cancer, 51.7 years in subjects with thyroid disease, and 48.0 years in those without thyroid disease. The proportions of female subjects were 87.9% and 86.4% in subjects with PTC and thyroid diseases, respectively, compared to 51.8% in subjects with no thyroid disease.
The daily energy intake was 1,333.6 kcal in patients with PTC, much lower than that in those with (1,863.7 kcal) or without (2,070.6 kcal) thyroid disease. Intake of the carbohydrate, protein, and fat macronutrients was significantly different across groups after adjusting for age, sex, and energy intake. Carbohydrate intake as a percentage of energy was 59.3% in subjects with PTC, which was lower than values for those with (66.8%) or without (65.9%) thyroid disease. Protein intake as a percentage of energy was 17.7% in patients with PTC, which was higher than those in subjects with (14.0%) or without (14.4%) thyroid disease.
Fig. 2 presents the distribution of UIC according to thyroid disease groups. In accordance with WHO recommendations, the prevalence of insufficient urinary iodine was 7.3% in subjects with PTC, which was lower than that in participants with (17.4%) or without (13.2%) thyroid disease. However, the prevalence of excessive iodine was 54.4% in patients with PTC, similar to subjects with thyroid disease (55.4%) and slightly higher than that in subjects without thyroid disease (47.7%).
Regarding UIC, as almost half of the study subjects excreted more than 300 μg/L of iodine, the distribution of subjects with excessive iodine was examined across groups. When participants with a UIC above 300 μg/L were further sub-grouped, the proportion with UIC >3,000 μg/L was 10.2% in subjects with PTC, which was higher than those with (7.6%) or without (4.8%) thyroid disease.
Fig. 3 presents the distribution of dietary iodine intake based on 24-hour recall according to thyroid disease groups. The median was 341.7 μg/day in patients with PTC, similar to those in subjects with (350.1 μg/day) and without (352.7 μg/day) thyroid disease. However, the distribution differed across groups. The interquartile range (25th to 75th percentile) was 191.2 to 743.0 μg/day in patients with PTC, a wider range than was found in subjects with (218.9 to 583.9 μg/day) or (209.2 to 620.5 μg/day) without thyroid disease.
In the evaluation of dietary iodine using DRIs for Koreans, the prevalence of iodine deficiency was 4.5% among patients with PTC, which was lower than that among subjects with (7.1%) or without (6.3%) thyroid disease. However, the prevalence of iodine excess was 7.2%, which was higher than that among subjects with (4.4%) or without (3.9%) thyroid disease.
Fig. 4 presents the major food groups that contributed to total iodine intake according to thyroid disease groups. The food group contributing most to total iodine intake in all groups was seaweed, but the proportion of seaweed relative to total iodine intake differed across groups. The proportion of iodine coming from seaweed was 79.5% in patients with PTC; this was much higher than the proportions among subjects with (39.6%) or without (55.8%) thyroid disease based on the KNHANES results, although data were obtained via the same 24-hour dietary recall method. The seaweed food group was followed by salted vegetables (including kimchi), fish and seashells, and milk and milk products in all groups.
Data on dietary iodine were obtained by two different methods from subjects with PTC. Fig. 5 presents the distribution of dietary iodine and the top 10 food items contributing to total iodine intake according to the method used.
The median dietary iodine was 341.7 μg/day according to the 24-hour recall and 405.9 μg/day according to the FFQ. The interquartile range for the 24-hour recall (191.2 to 743.0 μg/day) was broader than that for data obtained using the FFQ (271.4 to 621.9 μg/day).
The top food item was brown kelp (dried), which accounted for 57.5% of the total iodine intake according to 24-hour recall, and it was followed by sea mustard (dried+stem) (14.0%), kimchi (baechukimchi+kkakdugi) (5.1%), laver (3.4%), milk (2.1%), and eggs (1.4%). The top food items differed from those identified in the 24-hour recall because food items in the FFQ were preselected. For example, sea mustard and brown kelp were given as a single food item in the FFQ, and this contributed 23.3% of the total iodine intake according to the FFQ results. Certain foods are high in iodine content, so the top 10 food items accounted for 85% to 87% of the total iodine intake according to the 24-hour recall and FFQ.
Table 2 compares the iodine measurements according to thyroid disease groups. Altogether, 53.2% to 66.5% of the measurement pairs fell within ±1 quintile and 74.0% to 85.4% within ±2 quintiles. The highest percentage of agreement was 85.4% within ±2 quintiles between dietary iodine based on 24-hour recall and urinary iodine among subjects with PTC.
The correlation coefficients differed by measurement type. The highest correlation coefficient (0.4826 based on the Pearson method and 0.3783 based on the Spearman method), was between dietary iodine based on 24-hour recall and urinary iodine (I/Cr ratio) in patients with PTC after adjustment for age, sex, and energy intake. By contrast, the coefficient between dietary iodine based on 24-hour recall and urinary iodine among subjects with thyroid disease from the KNHANES was not significant, and that for participants without thyroid disease was low.
The correlations of dietary iodine calculated from the FFQ with urinary iodine or dietary iodine based on 24-hour recall were not significant.
In this study, we evaluated iodine status using dietary and urinary iodine among Korean adults with PTC. The prevalence of excessive iodine as measured by dietary and urinary iodine was higher in patients with PTC than Korean adults without thyroid disease from the KNHANES. The food group contributing the most to total iodine intake was seaweed. Urinary iodine was highly correlated with iodine intake as measured by 24-hour recall, but not with measurements made using the FFQ in subjects with PTC.
In our study, a higher proportion of PTC patients had excessive iodine status than those without thyroid disease. According to UIC, more than half of those with PTC exhibited excessive iodine concentrations (i.e., >300 μg/L). This finding is in agreement with previous studies, which showed an excessive UIC in 67.0% of patients with PTC compared to 19.9% in the control group [22] or in 44.3% of patients with PTC compared to 22.2% of patients without PTC [23]. A case-control study in China reported the median UIC was 517.2 μg/L in PTC patients compared to 194.3 μg/L in a control group [24], and another study in Korea reported a level of 786.0 μg/L in PTC patients compared to 112.0 μg/L in a control group [25].
Excessive urinary iodine was also found in other groups of Koreans, including 56% of patients with thyroid nodules [26] and 64% of preschool children [6]; these frequencies are markedly higher than the 9.0% of young adults in the US mountainous west [27] or 11.6% of adults on the Tibetan plateau [28]. This result suggests that half of the Korean population is at risk of excessive iodine status as determined by the UIC regardless of thyroid disease. In fact, the WHO criterion for “excessive” refers to any amount exceeding the level required to prevent and control iodine deficiency. Further research is needed to redefine the WHO criterion for UIC values above 300 μg/L to prevent and manage iodine excess.
The tolerable UL for iodine intake is set at 2,400 μg/day for Korean adults, which is far higher than the WHO “excessive” criterion. The UL refers to the highest level of usual iodine intake that is considered to pose no risk of adverse health effects in a healthy population. However, the UL for those with thyroid cancer or thyroid disorders has not yet been considered due to limited data on the relationship between dietary iodine and thyroid function. Furthermore, the current UL for iodine may not be appropriate for evaluating iodine status in terms of minimizing the risk of thyroid cancer or disorders. Nevertheless, we found in this study that the prevalence of excess iodine intake above the UL was 7.2% in subjects with PTC, almost double that in the KNHANES subjects with or without thyroid disease. The distribution of dietary iodine (interquartile range) was also broader in subjects with PTC than in Korean adults with or without thyroid disease from KNHANES.
In this study, the median dietary iodine was 341.7 to 352.7 μg/day, which is comparable to the 312 to 413 μg/day value found in Japanese adults [14] but almost double that in British and French women (150 to 190 μg/day) [15,16]. Considering that the RNI of iodine is 150 μg/day for Korean adults, most Korean adults have a higher dietary iodine intake than is recommended.
This variation in dietary iodine levels across countries is due to differences in dietary cultures. In particular, seaweed, which contains extremely high iodine content, is commonly consumed in countries such as Korea and Japan. Variations in the food source of dietary iodine among countries are clear in numerous reports: kelp and fish were the main contributors to Japanese iodine status [29], dairy and eggs were primary predictors of iodine status in United States adults [27], and dairy products (34.8%) and seafood (14.5%) were the main food groups providing iodine in French women [16].
In this study, the major contributor to total iodine intake was also seaweed. Furthermore, seaweed accounted for 79.5% of the total iodine intake in subjects with PTC, a much higher proportion than among Korean adults participating in the KNHANES. A cohort study of Japanese women reported that seaweed consumption was positively associated with an increased risk of thyroid cancer over a mean of 14.5 years [30]. However, another cohort study reported no association [31]. Thus, more studies are needed to confirm the effect of excessive iodine intake from seaweed on thyroid cancer.
The UIC in spot urine samples is regarded as a sensitive biomarker of excessive iodine intake [1], but the UIC alone does not adequately clarify the role of iodine intake in thyroid cancer or disorders in Koreans with iodine-rich diets. The UIC reflects recent iodine intake. Indeed, we found that dietary iodine intake as measured by 24-hour recall was highly correlated with urinary iodine in subjects with thyroid cancer, as 24-hour recall responses and urine samples were obtained on the same day in the hospital. The correlation in this study (r=0.43673) is comparable to that in a previous study on Japanese adults that showed a correlation of 0.37 between iodine intake calculated based on dietary records and urinary iodine when the dietary record and urine sample were obtained on the same day [29]. However, the correlation between dietary intake based on the 24-hour recall and urinary iodine was not significant in the KNHANES participants, likely because the 24-hour recall and urine collection were separately administered at different times.
However, in PTC patients, dietary iodine as assessed by the FFQ was not significantly correlated either with dietary iodine based on the 24-hour recall or the UIC, although the agreement between the two measurement methods was fairly good. Two primary methods are used to assess individual diets. A 24-hour recall or dietary record reflects recent intake and can identify all food items consumed; the FFQ, in contrast, reflects the respondent’s usual diet over the previous year and represents the dietary frequency of pre-selected food items.
To elucidate the role of dietary iodine in the development of thyroid cancer or disorders, an estimation of usual iodine intake is necessary. Usual intake or long-term iodine intake can be estimated by accumulating multiple days of 24-hour recall or dietary records or by administering validated FFQs. The FFQ employed in this study was developed to evaluate the overall nutrient intake of Korean adults for the Korean Genome Epidemiologic Study [19]; thus, it was not specific to iodine and did not include certain iodine-rich food items, such as seaweed, in detail.
Another concern about estimating usual iodine intake is seaweed consumption. As seaweed is not always consumed on a daily basis, the consumption of seaweed influences daily iodine intake, leading to exceptionally large intra-individual variance. Katagiri et al. [9] estimated the usual iodine intake in Japanese adults using 16 days of dietary records over four seasons. They reported that the median iodine intake of each subject was 1,031 μg/day, whereas the median iodine intake over all survey days for all subjects (1,920 days=120 subjects×16 days) was only 273 μg/day in men. This was because the subjects consumed iodine-rich foods (kelp or soup stock) on at least one or more days of the 16 days. A similar finding was also reported in Korea. When Korean adults from the KNHANES were divided into seaweed consumers and non-consumers, the median iodine intake among seaweed consumers was 495.7 μg/day, compared to 241.2 μg/day in non-consumers [10].
Our study has several limitations. First, the patients with PTC were recruited from a hospital, whereas data for the comparison groups comprising subjects with or without thyroid disease were obtained from the KNHANES. It is possible that these comparisons could have been biased due to differences among the subject groups. However, both studies employed the same methods to assess dietary iodine and analyze urinary iodine, and KNHANES participants are representative of the Korean population. Given the limited data available on dietary iodine intake in Koreans differentiated by thyroid disease, this study contributes to the development of basic evidence for this population.
Second, iodine intake was calculated using the iodine database newly established by Choi et al. [10]. Due to the lack of a national-level iodine database, it is possible that the iodine intake values could have misrepresented the true values. A national-level iodine database is needed to estimate dietary iodine accurately. Third, although the same dietary assessment of a single 24-hour recall was used to estimate dietary intake in PTC patients and Korean adults from KNHANES, the 24-hour recall in PTC patients was administered about foods before dinner due to fasting for surgery. This led to significant differences in the intake of all nutrients between the PTC patients and the control groups from the KNHANES. A single day of 24-hour recall cannot reflect usual nutrient intake; as such, this study did not account for between-group differences in nutritional status. However, PTC patients showed higher dietary iodine intake than those in the control groups even after adjusting for energy intake. To compare dietary iodine accurately, it is necessary to assess recall responses from multiple days or to develop an iodine-specific FFQ in future studies.
Finally, this study employed a cross-sectional design, so we could not evaluate the causal relationships between dietary iodine and thyroid disease. Further longitudinal studies are needed to confirm this relationship.
Our study also has several strengths. This study provided data on both dietary iodine and urinary iodine to evaluate the iodine status of patients with PTC. In addition, dietary iodine intake was assessed using 24-hour recall and an FFQ, and major food sources of iodine were identified. To the best of our knowledge, this is the first study to evaluate the iodine status in patients with PTC using both urinary iodine and dietary iodine intake as assessed by two different methods. Our study provides baseline data that can aid in understanding the impacts of iodine-rich diets in Korean adults according to thyroid disease status, and the findings will be useful for developing dietary guidelines for iodine intake in subjects with thyroid disease.
In conclusion, the prevalence of iodine excess as assessed by both dietary and urinary iodine was higher in subjects with PTC than in Korean adults with or without thyroid disease from the KNHANES. The contribution of seaweed to total iodine was also higher in subjects with PTC. To elucidate the role of iodine intake in thyroid disorders in Koreans, further studies that estimate usual iodine intake using various measures and evaluate its association with thyroid disorders in a longitudinal study are needed.
ACKNOWLEDGMENTS
This study was supported by grants from the Catholic University of Korea and the Research Fund 2021 and the Gachon University Gil Medical Center (FRD2017-17).
REFERENCES
1. Farebrother J, Zimmermann MB, Andersson M. Excess iodine intake: sources, assessment, and effects on thyroid function. Ann N Y Acad Sci. 2019; 1446:44–65.

2. Laurberg P, Jorgensen T, Perrild H, Ovesen L, Knudsen N, Pedersen IB, et al. The Danish investigation on iodine intake and thyroid disease, DanThyr: status and perspectives. Eur J Endocrinol. 2006; 155:219–28.

3. Yu X, Fan C, Shan Z, Teng X, Guan H, Li Y, et al. A five-year follow-up study of goiter and thyroid nodules in three regions with different iodine intakes in China. J Endocrinol Invest. 2008; 31:243–50.

4. Lee JH, Hwang Y, Song RY, Yi JW, Yu HW, Kim SJ, et al. Relationship between iodine levels and papillary thyroid carcinoma: a systematic review and meta-analysis. Head Neck. 2017; 39:1711–8.

5. Kim HI, Oh HK, Park SY, Jang HW, Shin MH, Kim SW, et al. Urinary iodine concentration and thyroid hormones: Korea National Health and Nutrition Examination Survey 2013–2015. Eur J Nutr. 2019; 58:233–40.

6. Lee J, Kim JH, Lee SY, Lee JH. Iodine status in Korean preschool children as determined by urinary iodine excretion. Eur J Nutr. 2014; 53:683–8.

7. Kim S, Kwon YS, Kim JY, Hong KH, Park YK. Association between iodine nutrition status and thyroid disease-related hormone in Korean adults: Korean National Health and Nutrition Examination Survey VI (2013–2015). Nutrients. 2019; 11:2757.

8. Carriquiry AL, Spungen JH, Murphy SP, Pehrsson PR, Dwyer JT, Juan W, et al. Variation in the iodine concentrations of foods: considerations for dietary assessment. Am J Clin Nutr. 2016; 104(Suppl 3):877S–87S.

9. Katagiri R, Asakura K, Sasaki S, Hirota N, Notsu A, Miura A, et al. Estimation of habitual iodine intake in Japanese adults using 16 d diet records over four seasons with a newly developed food composition database for iodine. Br J Nutr. 2015; 114:624–34.

10. Choi JY, Ju DL, Song Y. Revision of an iodine database for Korean foods and evaluation of dietary iodine and urinary iodine in Korean adults using 2013–2015 Korea National Health and Nutrition Examination Survey. J Nutr Health. 2020; 53:271.

11. Vandevijvere S, Lin Y, Moreno-Reyes R, Huybrechts I. Simulation of total dietary iodine intake in Flemish preschool children. Br J Nutr. 2012; 108:527–35.

12. Cao LZ, Peng XD, Xie JP, Yang FH, Wen HL, Li S. The relationship between iodine intake and the risk of thyroid cancer: a meta-analysis. Medicine (Baltimore). 2017; 96:e6734.
13. Cho YA, Kim J. Dietary factors affecting thyroid cancer risk: a meta-analysis. Nutr Cancer. 2015; 67:811–7.

14. Imaeda N, Kuriki K, Fujiwara N, Goto C, Tokudome Y, Tokudome S. Usual dietary intakes of selected trace elements (Zn, Cu, Mn, I, Se, Cr, and Mo) and biotin revealed by a survey of four-season 7-consecutive day weighed dietary records in middle-aged Japanese dietitians. J Nutr Sci Vitaminol (Tokyo). 2013; 59:281–8.

15. Combet E, Bouga M, Pan B, Lean ME, Christopher CO. Iodine and pregnancy: a UK cross-sectional survey of dietary intake, knowledge and awareness. Br J Nutr. 2015; 114:108–17.

16. Mancini FR, Rajaobelina K, Dow C, Habbal T, Affret A, Balkau B, et al. High iodine dietary intake is associated with type 2 diabetes among women of the E3N-EPIC cohort study. Clin Nutr. 2019; 38:1651–6.

17. Combet E, Lean ME. Validation of a short food frequency questionnaire specific for iodine in U.K. females of childbearing age. J Hum Nutr Diet. 2014; 27:599–605.

18. Kweon S, Kim Y, Jang MJ, Kim Y, Kim K, Choi S, et al. Data resource profile: the Korea National Health and Nutrition Examination Survey (KNHANES). Int J Epidemiol. 2014; 43:69–77.

19. Ahn Y, Kwon E, Shim JE, Park MK, Joo Y, Kimm K, et al. Validation and reproducibility of food frequency questionnaire for Korean genome epidemiologic study. Eur J Clin Nutr. 2007; 61:1435–41.

20. The Korean Nutrition Society. Dietary reference intakes for Koreans 2020. Sejong: Ministry of Health and Welfare;2020. p. 399.
21. World Health Organization. Assessment of iodine deficieny disorders and monitoring their elimination. Geneva: WHO;2007. p. 98.
22. Wang F, Wang Y, Wang L, Wang X, Sun C, Xing M, et al. Strong association of high urinary iodine with thyroid nodule and papillary thyroid cancer. Tumour Biol. 2014; 35:11375–9.

23. Huang F, Cong W, Xiao J, Zhou Y, Gong M, Sun J, et al. Association between excessive chronic iodine exposure and the occurrence of papillary thyroid carcinoma. Oncol Lett. 2020; 20:189.

24. Zhang L, Fang C, Liu L, Liu X, Fan S, Li J, et al. A case-control study of urinary levels of iodine, perchlorate and thiocyanate and risk of papillary thyroid cancer. Environ Int. 2018; 120:388–93.

25. Lee JH, Song RY, Yi JW, Yu HW, Kwon H, Kim SJ, et al. Case-control study of papillary thyroid carcinoma on urinary and dietary iodine status in South Korea. World J Surg. 2018; 42:1424–31.

26. Kim HJ, Kim NK, Park HK, Byun DW, Suh K, Yoo MH, et al. Strong association of relatively low and extremely excessive iodine intakes with thyroid cancer in an iodine-replete area. Eur J Nutr. 2017; 56:965–71.

27. Gostas DE, Larson-Meyer DE, Yoder HA, Huffman AE, Johnson EC. Dietary relationship with 24 h urinary iodine concentrations of young adults in the mountain west region of the United States. Nutrients. 2020; 12:121.

28. Ning P, Ren Q, Teng D, Zhang Z, Lv X, Meng S, et al. Current iodine nutrition status and prevalence of thyroid disorders in Tibetan adults in an oxygen-deficient plateau, Tibet, China: a population-based study. Thyroid. 2020; 30:759–66.

29. Katagiri R, Asakura K, Uechi K, Masayasu S, Sasaki S. Iodine excretion in 24-hour urine collection and its dietary determinants in healthy Japanese adults. J Epidemiol. 2016; 26:613–21.

30. Michikawa T, Inoue M, Shimazu T, Sawada N, Iwasaki M, Sasazuki S, et al. Seaweed consumption and the risk of thyroid cancer in women: the Japan Public Health Center-based Prospective Study. Eur J Cancer Prev. 2012; 21:254–60.
31. Wang C, Yatsuya H, Li Y, Ota A, Tamakoshi K, Fujino Y, et al. Prospective study of seaweed consumption and thyroid cancer incidence in women: the Japan collaborative cohort study. Eur J Cancer Prev. 2016; 25:239–45.
Fig. 1
Flowchart of subject selection process to evaluate iodine status using data from the Gachon University Gil Medical Center and the 2013–2015 Korea National Health and Nutrition Examination Survey (KNHANES). FFQ, food frequency questionnaire; BMI, body mass index.
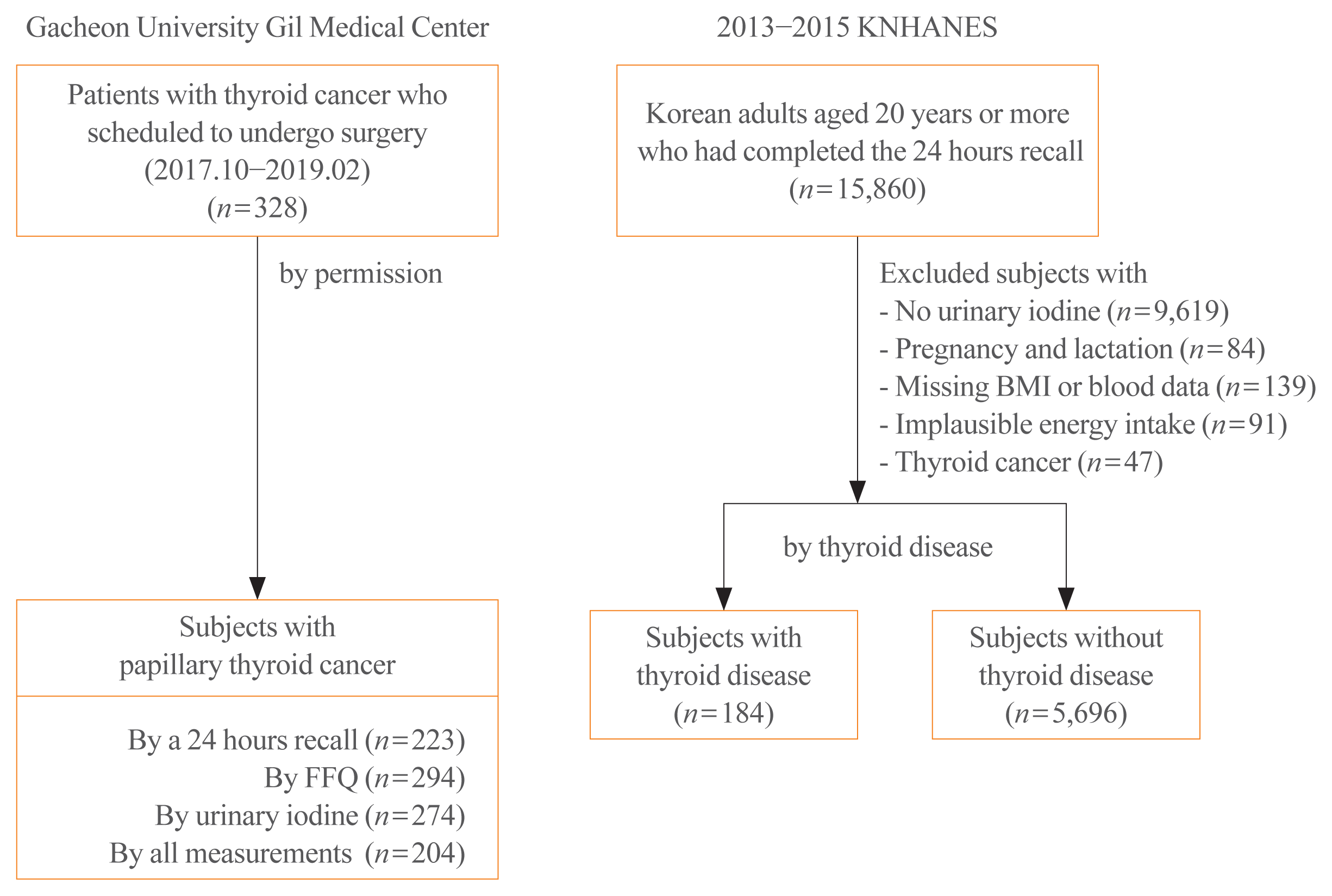
Fig. 2
Distribution of urinary iodine concentrations according to thyroid disease groups in Korean adults. (A) The percentage of subjects by World Health Recommendations and (B) by further categories among subjects with excessive urinary iodine (>300 μg/L). WHO, World Health Organization.
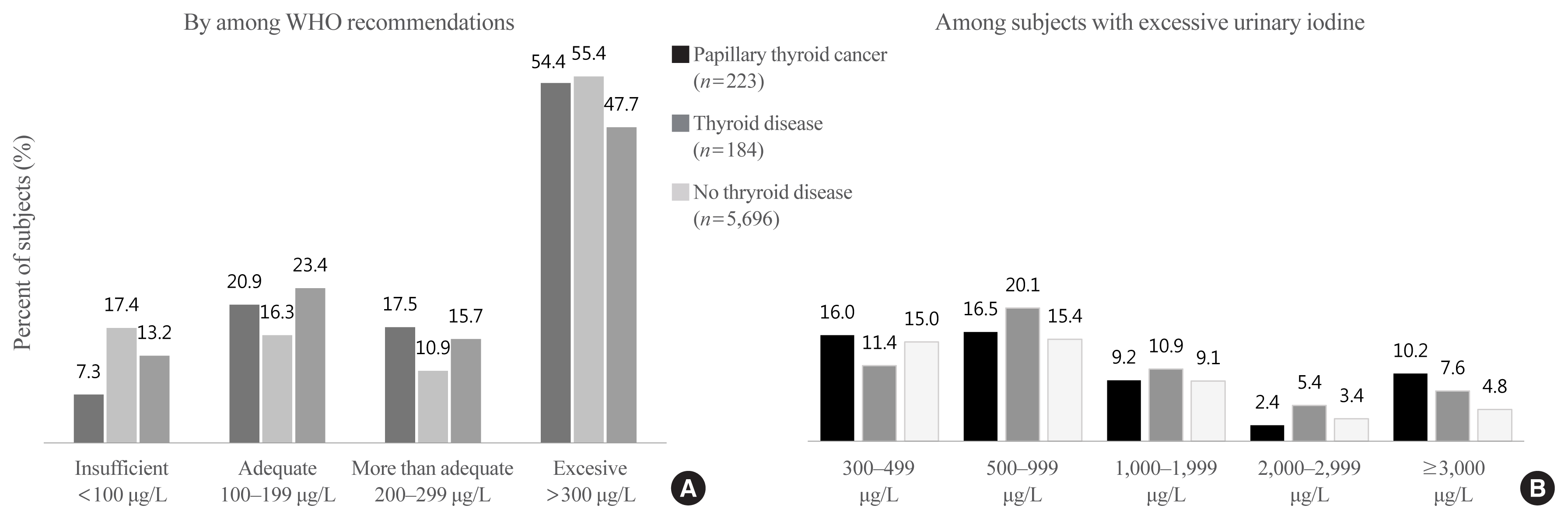
Fig. 3
Distribution of dietary iodine and evaluation of excessive iodine intake by dietary reference intakes for Koreans according to thyroid disease groups in Korean adults. (A) The distribution of dietary iodine intake and (B) the percentage of subjects who consumed less than the estimated average requirement (EAR) or more than the tolerable upper intake level (UL) of iodine. Q1: 25th percentile, Q3: 75th percentile, P90: 90th percentile, box: Q3-Q1 (interquartile range), ⋄: mean.
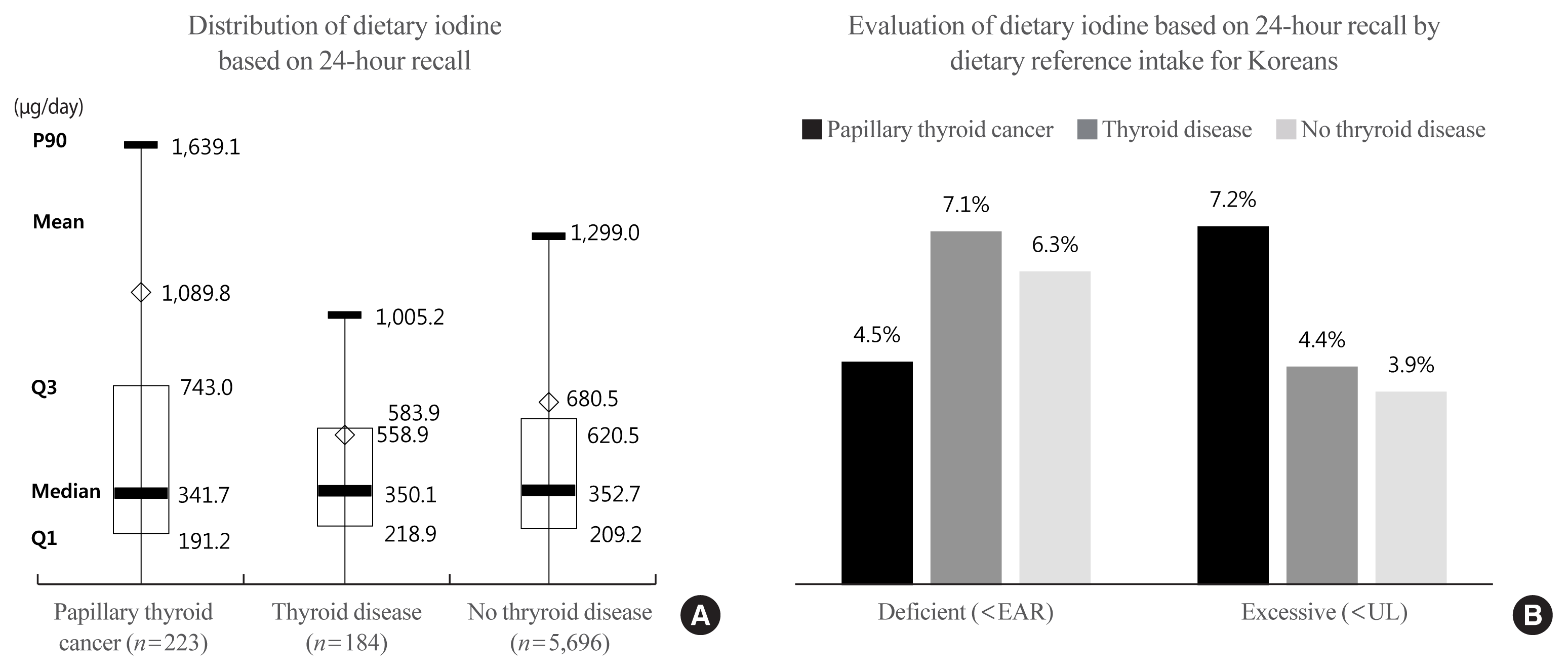
Fig. 4
The contribution of major food groups to total iodine intake based on 24-hour recall among subjects according to thyroid disease groups.
Fig. 5
Dietary iodine intake and the major food items contributing to total iodine intake by different dietary assessment methods in patients with papillary thyroid cancer. aBox and whisker plot; Q1: 25th percentile, Q3: 75th percentile, P90: 90th percentile, box: Q3-Q1 (interquartile range), ⋄: mean.
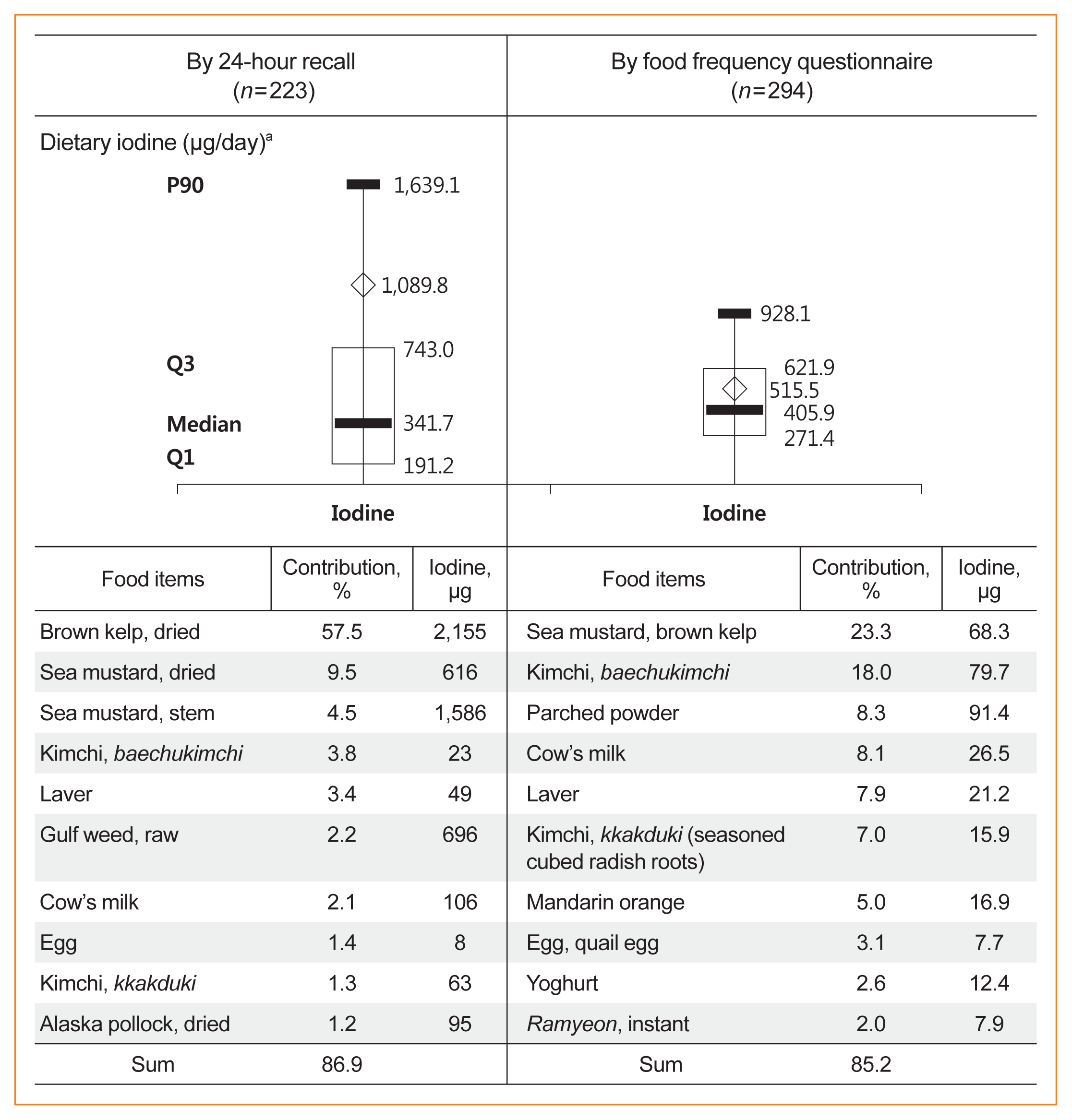
Table 1
The Basic Characteristics and Nutrient Intake by 24-Hour Recall in Korean Adults According to Thyroid Disease Groups
| Characteristic | Hospital | 2013–2015 KNHANES | P valuea | |
|---|---|---|---|---|
|
|
|
|||
| Papillary thyroid cancer (n=223) | Thyroid disease (n=184) | No thyroid disease (n=5,696) | ||
| Age, yr | 48.9±12.7 | 51.7±12.2 | 48.0±15.4 | <0.0001 |
|
|
||||
| Sex | <0.0001 | |||
| Men | 27 (12.1) | 25 (13.6) | 2,746 (48.2) | |
| Women | 196 (87.9) | 159 (86.4) | 2,950 (51.8) | |
|
|
||||
| BMI, kg/m2 | 24.4±5.7b | 23.6±3.2 | 23.9±3.5 | 0.0013 |
|
|
||||
| Energy, kcal | 1,333.6±515.4 | 1,863.7±669.8 | 2,070.6±833.1 | <0.0001 |
|
|
||||
| Carbohydrate, g | 203.8±77.5 | 301.1±106.2 | 317.6±123.2 | <0.0001 |
|
|
||||
| Protein, g | 77.6±267.2 | 64.6±35.7 | 71.4±37.2 | <0.0001 |
|
|
||||
| Fat, g | 37.9±27.3 | 40.5±30.9 | 44.7±32.1 | <0.0001 |
|
|
||||
| Energy from | ||||
| Carbohydrate, % | 59.3 (10.6) | 66.8 (11.6) | 65.9 (11.6) | <0.0001 |
| Protein, % | 17.7 (6.1) | 14.0 (4.1) | 14.4 (4.3) | <0.0001 |
| Fat, % | 23.0 (8.5) | 19.2 (9.8) | 19.6 (9.4) | <0.0001 |
|
|
||||
| Calcium, mg | 400.3±240.2 | 479.4±268.9 | 496.5±285.9 | <0.0001 |
|
|
||||
| Phosphate, mg | 786.6±322.7 | 1,037.2±476.2 | 1,100.3±495.0 | 0.0795 |
|
|
||||
| Iron, mg | 12.0±6.3 | 17.1±15.3 | 18.5±48.9 | 0.9795 |
|
|
||||
| Sodium, mg | 3,864.7±2,060.9 | 3,667.6±2,203.4 | 3,929.2±2,486.3 | <0.0001 |
|
|
||||
| Potassium, mg | 1,930.5±804.2 | 3,069.6±1,357.4 | 3,130.1±1,544.8 | <0.0001 |
|
|
||||
| Vitamin A, μgRE | 559.7±558.4 | 719.2±673.2 | 764.5±1,084.4 | 0.9433 |
|
|
||||
| Thiamin, mg | 0.8±0.4 | 1.9±0.8 | 2.1±1.0 | <0.0001 |
|
|
||||
| Riboflavin, mg | 0.9±0.5 | 1.3±0.7 | 1.4±0.8 | 0.2119 |
|
|
||||
| Vitamin C, mg | 72.0±91.7 | 121.1±125.5 | 107.4±124.3 | 0.0456 |
Table 2
Agreement and Correlations between Dietary Iodine and Urinary Iodine According to Thyroid Disease Groups in Korean Adults
| Variable | Agreement | Correlationa | ||
|---|---|---|---|---|
|
|
|
|||
| ±1 quintile | ±2 quintile | Pearson | Spearman | |
| Subjects with papillary thyroid cancer from hospital | ||||
| Dietary by 24-hour recall vs. urinary iodineb (n=206) | 66.5 | 85.4 | 0.4826 (<0.0001) | 0.3783 (<0.0001) |
| Dietary by FFQ vs. urinary iodineb (n=248) | 53.6 | 78.2 | −0.1243 (0.0521) | 0.1006 (0.1163) |
| Dietary by FFQ vs. by 24-hour recall (n=204) | 54.4 | 79.4 | 0.1076 (0.1201) | 0.0725 (0.2959) |
|
|
||||
| Subjects with thyroid disease from KNHANES | ||||
| Dietary by 24-hour recall vs. urinary iodineb (n=184) | 53.2 | 74.0 | 0.0369 (0.5799) | 0.0155 (0.8159) |
| Subjects with no thyroid disease from KNHANES | ||||
| Dietary by 24-hour recall vs. urinary iodineb (n=5,696) | 54.5 | 78.2 | 0.0983 (<0.0001) | 0.0966 (<0.0001) |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download