INTRODUCTION
METHODS
Animals
Materials
Islet isolation procedure
Islet microencapsulation procedure
Confirm of microcapsules morphology and pore size
In vitro dexamethasone 21-phosphate release test
Microencapsulated islets viability measurement
Glucose stimulated insulin secretion test
Transplantation in mouse model
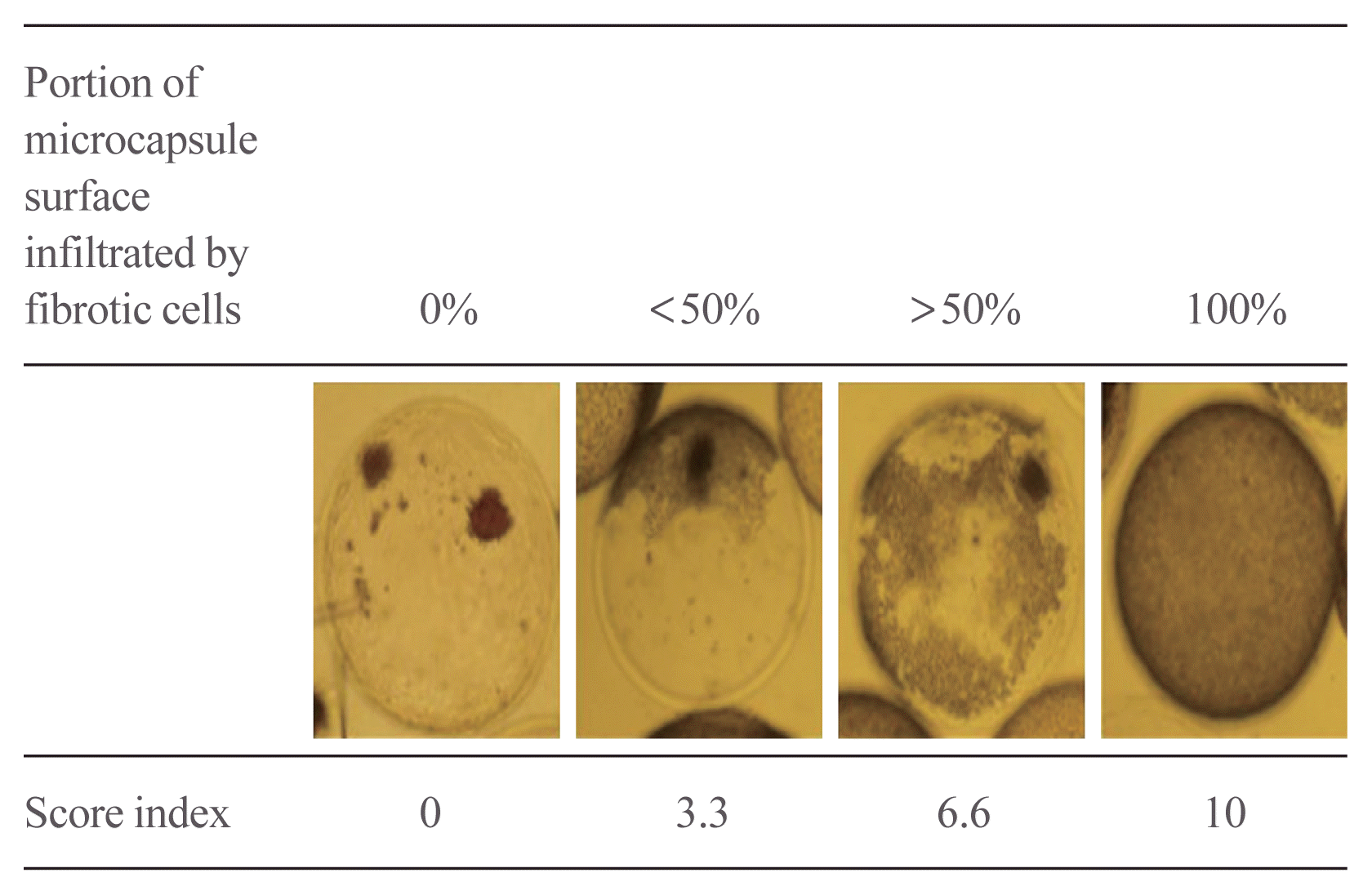
Fibrosis score calculate
Immunohistochemistry and Masson-Trichrome stain
Statistical analysis
RESULTS
Characterization of Dexa-chitosan-coated alginate microcapsules
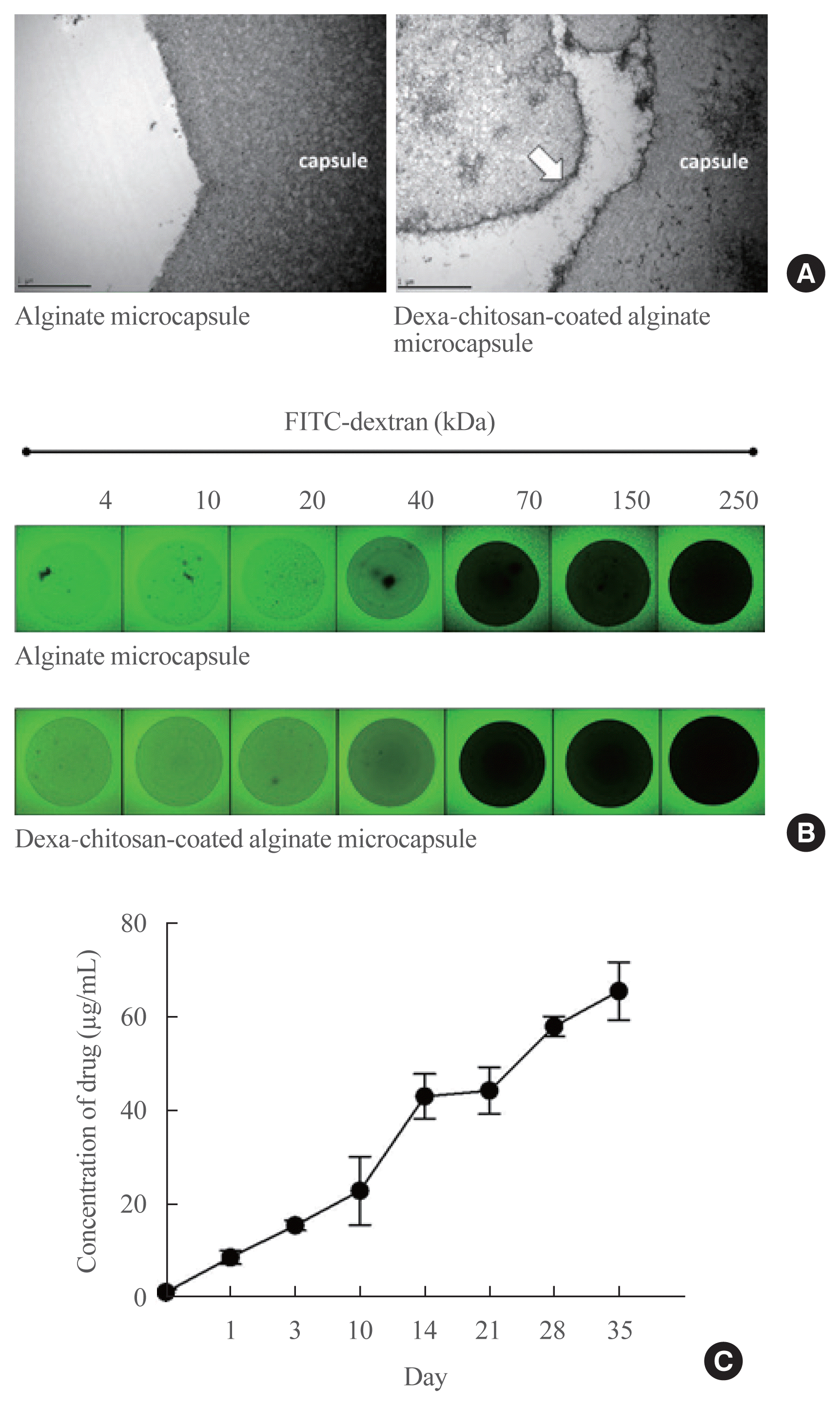 | Fig. 2Characterization of dexamethasone (Dexa)-chitosan-coated alginate microcapsules. (A) Observation of the microcapsule surface by transmission electron microscopy. The left panel is the alginate microcapsule (×10,000 magnification), and the right panel is the Dexa-chitosan-coated alginate microcapsule (white arrow; dexa-chitosan layer). (B) Observation of the diffusion and permeability assessment of microcapsules by fluorescein isothiocyanate (FITC)-labeled dextran. The molecular cutoff of each microcapsule type was between 40 and 70 kDa. (C) Dexa concentration for 36 days after incubation of 1,000 ea Dexa-chitosan-coated alginate microcapsules. |
Viability and function of microencapsulated islets
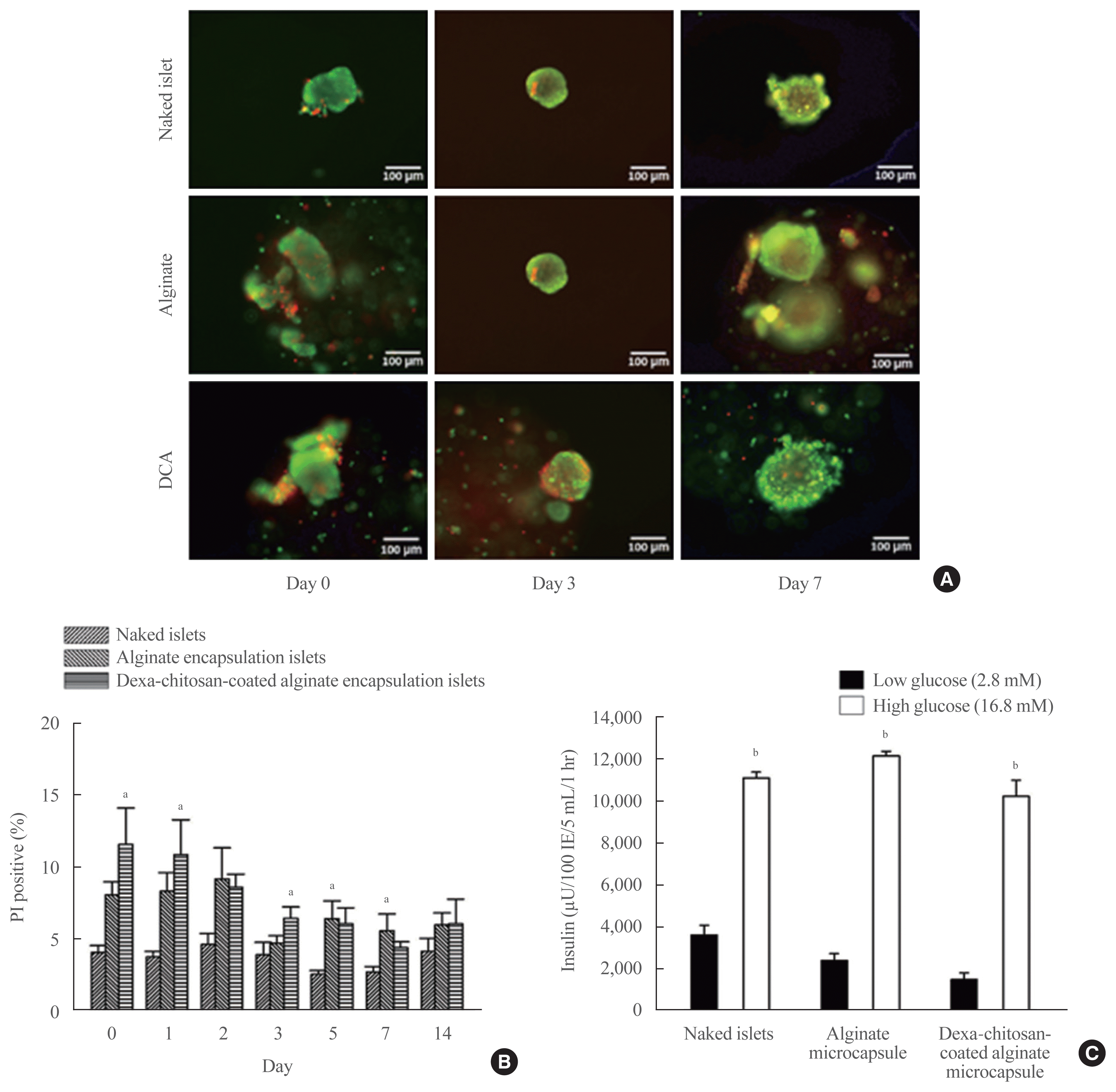 | Fig. 3Viability and secretory function of the microencapsulation islets. (A) Viability of microencapsulated islets based on acridine orange/propidium iodide (AO/PI) staining of naked islets, alginate microcapsules, and dexamethasone (Dexa)-chitosan-coated alginate microcapsules (DCAs) at days 0, 3, and 7. Scale bar=100 μm. (B) PI-positive cells (%) in naked islets, alginate microencapsulated islets and Dexa-chitosan-coated alginate microencapsulated islets over 14 days. (C) Insulin secretory function of microencapsulated islets during glucose-stimulated insulin secretion. Insulin secretion was significantly increased in response to glucose in all groups. aP<0.05 compared with the naked islets in each date group; bP<0.01. |
Improvement of blood glucose levels after Dexa-chitosan-coated alginate microencapsulated islet transplantation in diabetic mice
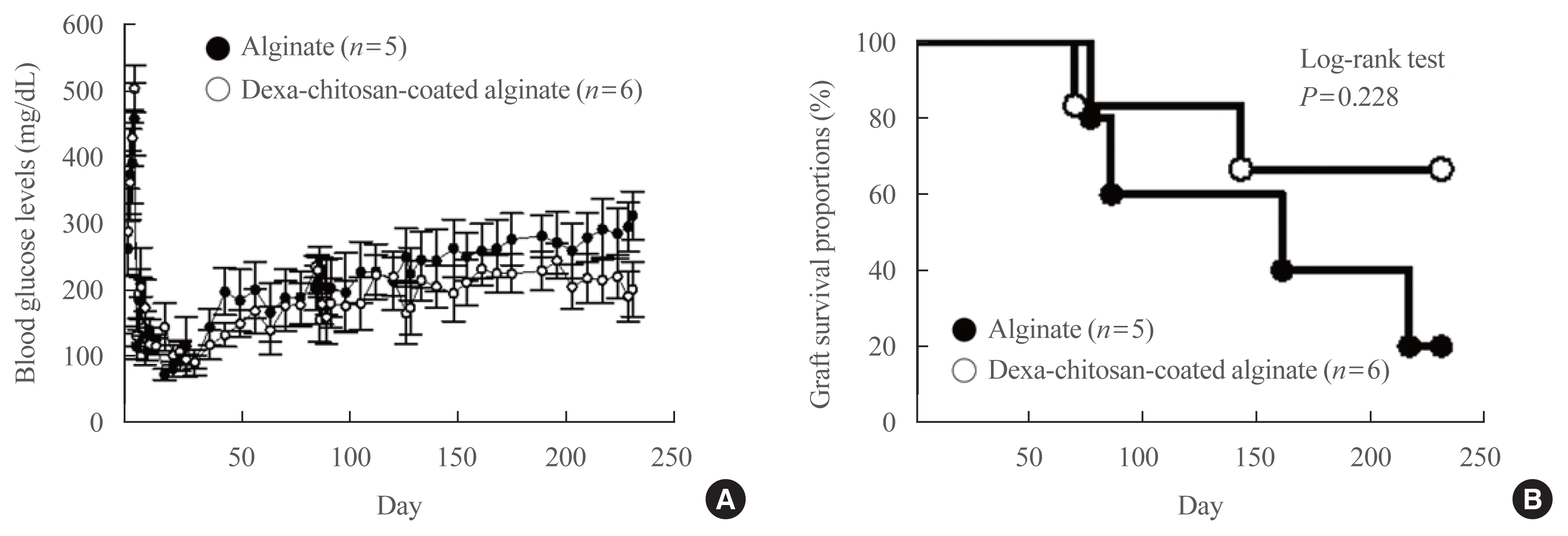 | Fig. 4Blood glucose level and graft survival in galactosyltransferase knock-out mice (GalT KO) mice transplanted with microencapsulated islets. (A) Change in blood glucose levels in GalT KO mice transplanted with alginate microencapsulated islets (n=5) and dexamethasone (Dexa)-chitosan coated alginate microencapsulated islets (n=6). (B) Graft survival proportions in GalT KO mice transplanted with microencapsulated islets. Graft survival proportions of Dexa-chitosan-coated alginate microencapsulated islets (open circle) improved approximately 60% compared with 20% survival for alginate microencapsulated islets (close circle) (log-rank test, P=0.228). |
Improved fibrosis of Dexa-chitosan-coated alginate microcapsules after transplantation
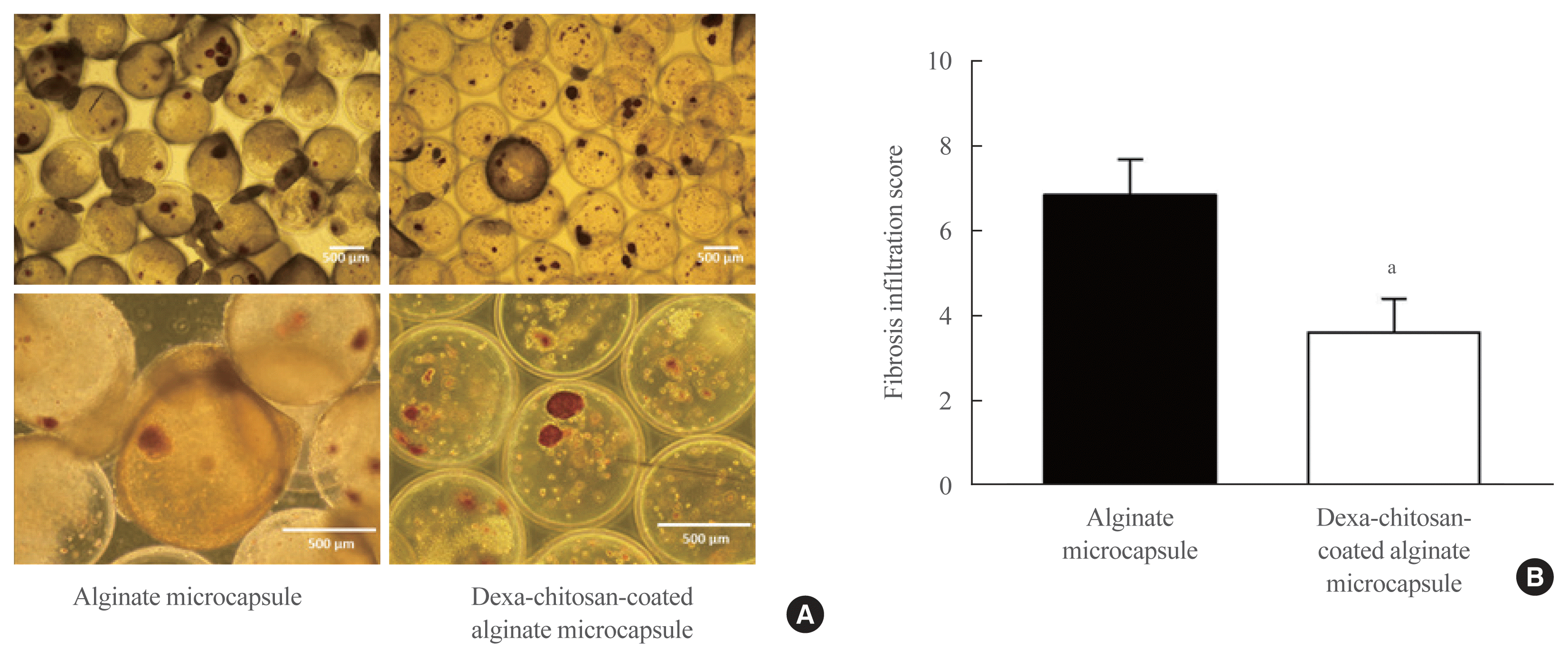 | Fig. 5Dithizone staining of harvested microencapsulated porcine islets and the measurement of fibrotic cell infiltration to the microcapsule surface. (A) The left panel shows harvested porcine islets with alginate microcapsules, and the right panel shows harvested dexamethasone (Dexa)-chitosan-coated alginate microcapsules. (B) Fibrosis infiltration score of microcapsules compared with alginate- and Dexa-chitosan-coated alginate microcapsules at 231 days after 10,000 IEq islet transplantation. aP<0.05 compared with alginate microcapsules. |
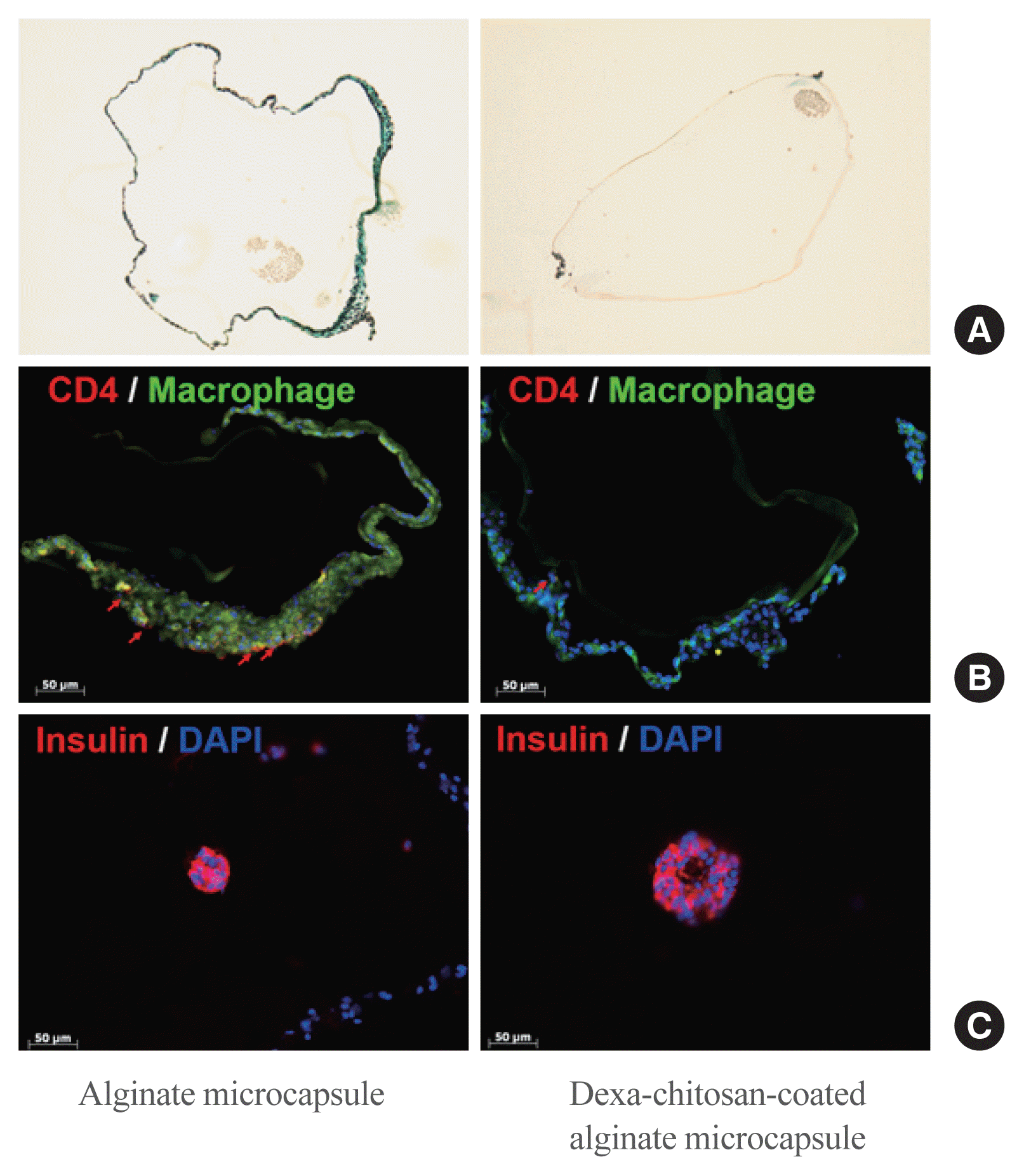 | Fig. 6Trichrome or immunohistochemistry staining of harvested encapsulated porcine islets and measurement of fibrotic cell infiltration to the microcapsule surface. (A) Fibrosis infiltrated stain (green) harvested from alginate microcapsules and dexamethasone (Dexa)-chitosan-coated alginate microcapsules. (B) Macrophages (green) and CD3 (red) were stained in alginate microcapsules. Red arrows indicate CD3-positive cells. Scale bar=50 μm. (C) Porcine islets were stained with insulin antibody (red). Scale bar=50 μm. DAPI, 4′-6-diamidino-2-phenylindole. |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download