INTRODUCTION
METHODS
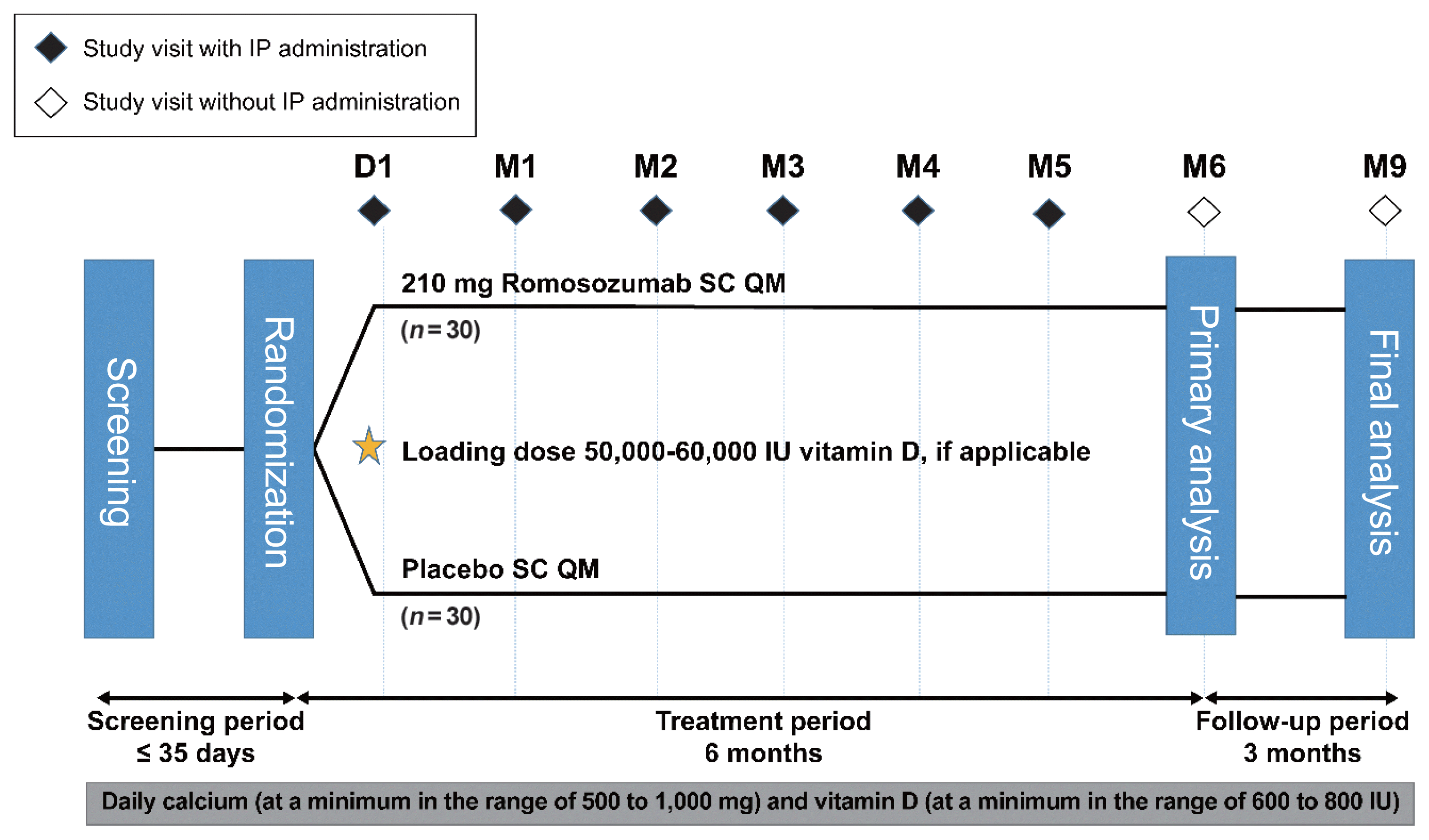
Study design
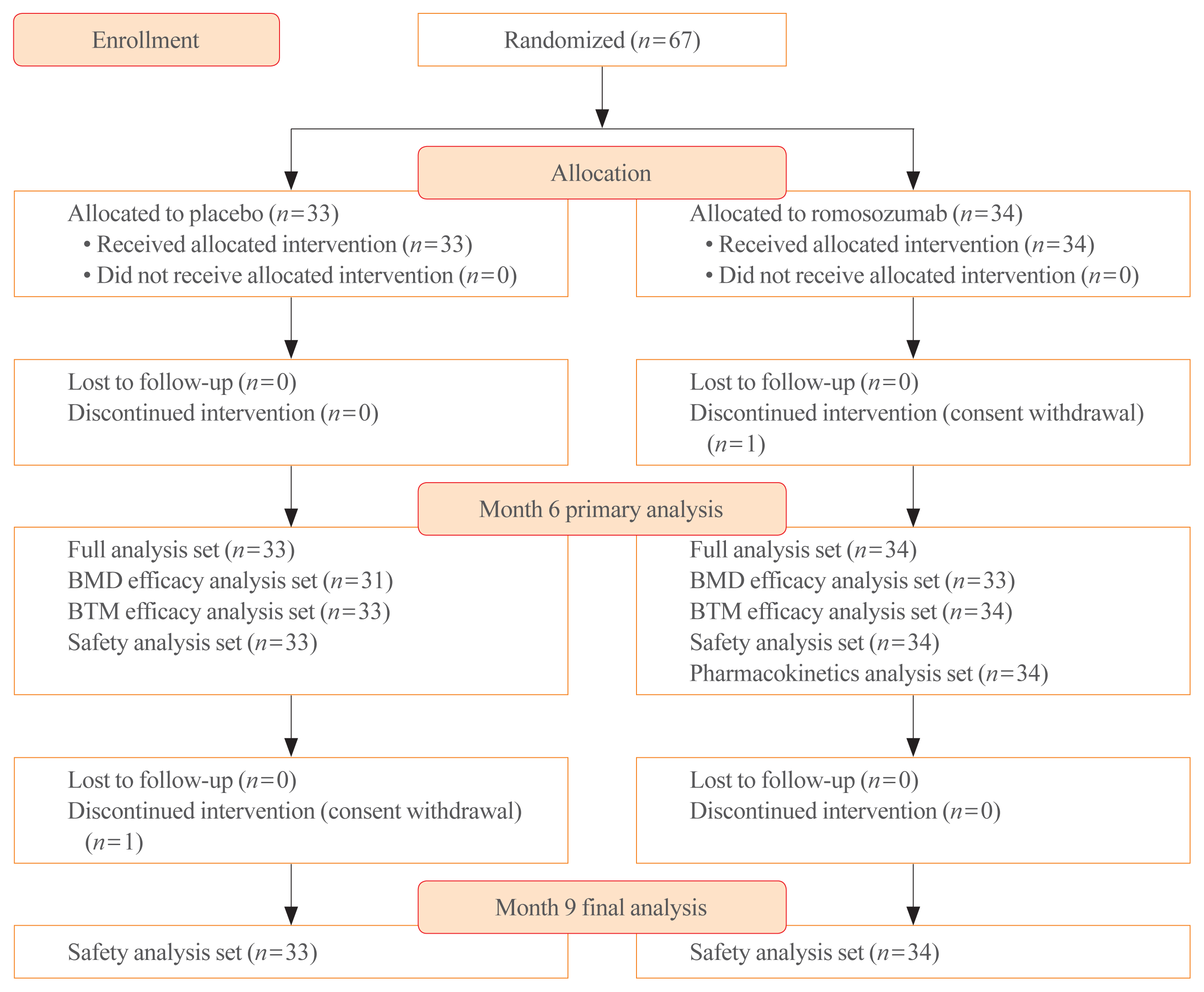
Participants
Randomization and blinding
Interventions
Outcomes
Sample size
Statistical methods
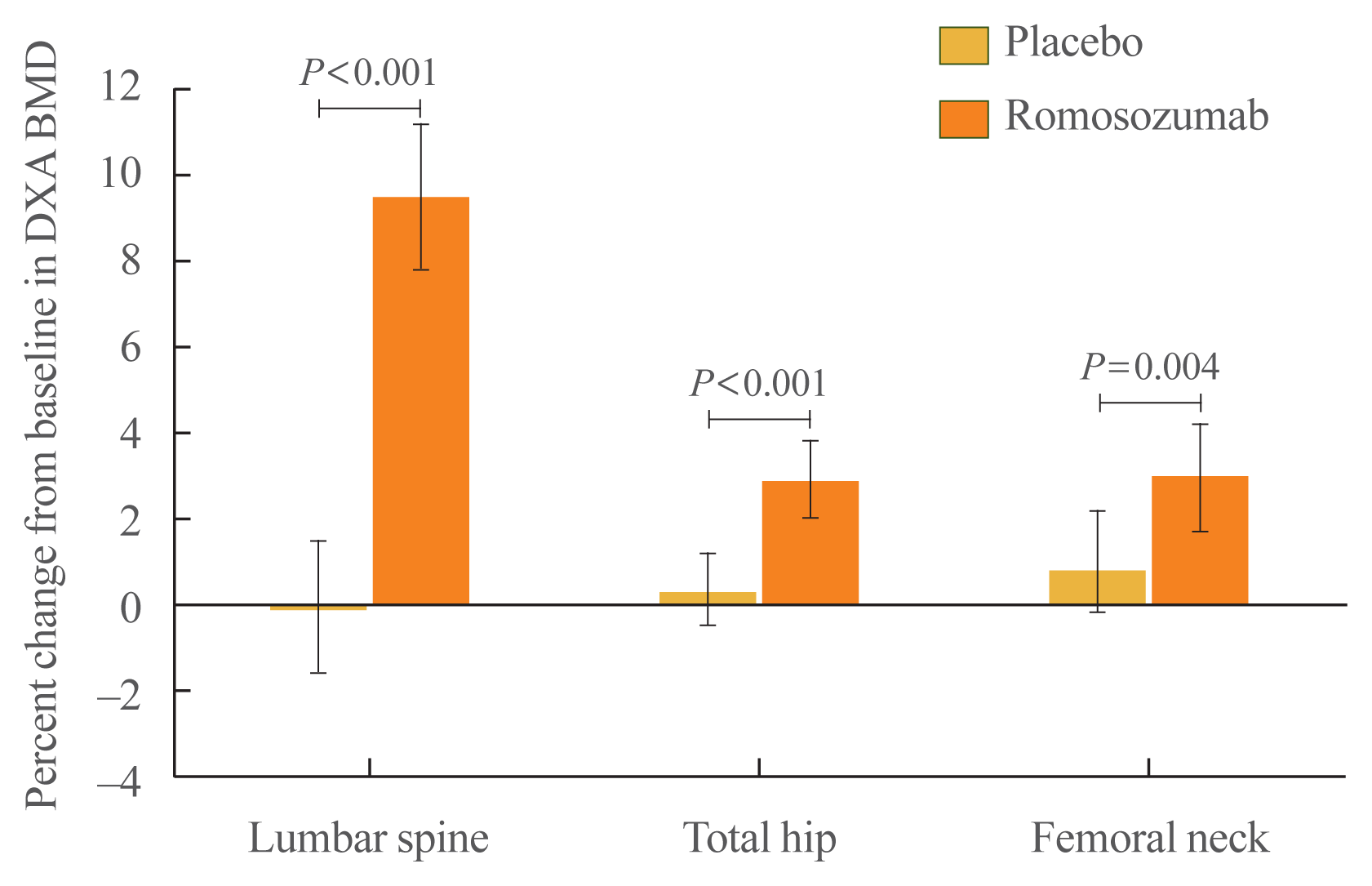
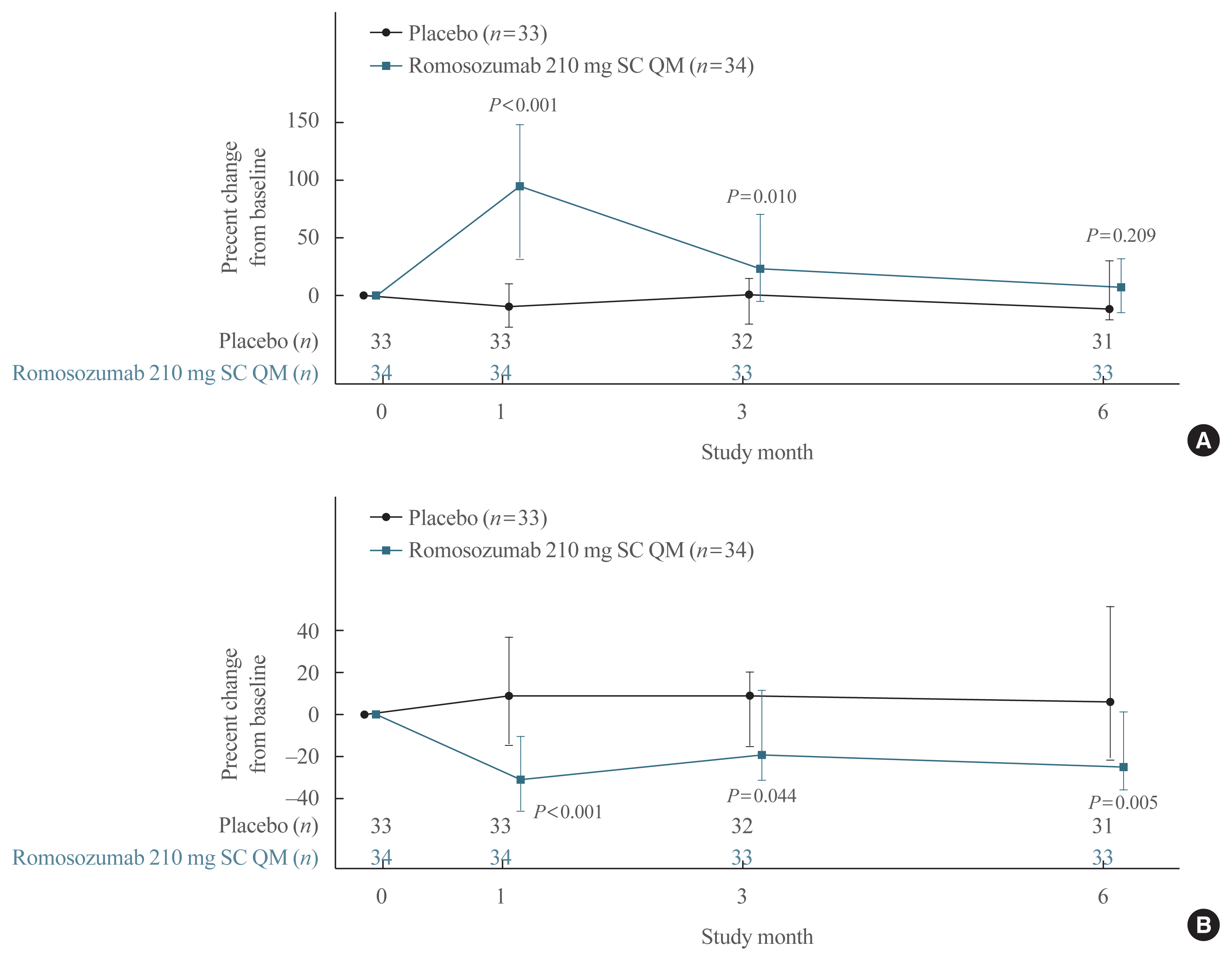
RESULTS
Patient demographics and baseline characteristics
Table 1
| Variable | Placebo (n=33) | Romosozumab 210 mg SC QM (n=34) | All (n=67) |
|---|---|---|---|
| Age, yr | 68.4±7.2 | 66.7±7.6 | 67.5±7.4 |
|
|
|||
| Age group, yr | |||
| <65 | 13 (39.4) | 14 (41.2) | 27 (40.3) |
| ≥65–<75 | 13 (39.4) | 16 (47.1) | 29 (43.3) |
| ≥75 | 7 (21.2) | 4 (11.8) | 11 (16.4) |
|
|
|||
| BMI, kg/m2 | 22.8±2.3 | 23.4±2.9 | 23.1±2.6 |
|
|
|||
| Historical fracture | |||
| Any | 8 (24.2) | 7 (20.6) | 15 (22.4) |
| Osteoporotica | 6 (18.2) | 5 (14.7) | 11 (16.4) |
| Nonvertebral | 5 (15.2) | 5 (14.7) | 10 (14.9) |
| Major nonvertebralb | 5 (15.2) | 4 (11.8) | 9 (13.4) |
|
|
|||
| Any historical fracture at or after the age of 45 years | |||
| Any | 8 (24.2) | 7 (20.6) | 15 (22.4) |
| Osteoporotica | 6 (18.2) | 5 (14.7) | 11 (16.4) |
| Nonvertebral | 5 (15.2) | 5 (14.7) | 10 (14.9) |
| Major nonvertebralb | 5 (15.2) | 4 (11.8) | 9 (13.4) |
|
|
|||
| Time since the most recent fracture, moc | |||
| <12 | 0 | 0 | 0 |
| ≥12 | 8 (24.2) | 7 (20.6) | 15 (22.4) |
|
|
|||
| BMD T-score | |||
| Lumbar spine | −2.6±0.6 | −2.7±0.8 | −2.7±0.7 |
| Total hip | −2.2±0.6 | −2.2±0.7 | −2.2±0.7 |
| Femoral neck | −2.5±0.5 | −2.4±0.6 | −2.5±0.6 |
|
|
|||
| Serum 25(OH) vitamin D, ng/mL | 29.5±5.7 | 31.3±8.9 | 30.4±7.5 |
|
|
|||
| Serum 25(OH) vitamin D level, ng/mL | |||
| <20 | 0 | 1 (2.9) | 1 (1.5) |
| ≥20 | 33 (100.0) | 33 (97.1) | 66 (98.5) |
Values are expressed as mean±standard deviation or number (%). Percentages are based on the number of patients randomized.
SC, subcutaneous; QM, once monthly; BMI, body mass index; BMD, bone mineral density; 25(OH), 25-hydroxyvitamin.
a An osteoporotic fracture is defined as a fracture reported on the fracture electronic case report form (eCRF) page, excluding “skull fracture,” “facial bones fracture,” “fingers fracture,” “toes fracture,” fractures with high trauma severity, and pathological fractures;




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download