Abstract
Background
Glucagon-like peptide-1 (GLP-1) analogues regulate glucose homeostasis and have anti-inflammatory properties, but cause gastrointestinal side effects. The fibroblast growth factor 21 (FGF21) is a hormonal regulator of lipid and glucose metabolism that has poor pharmacokinetic properties, including a short half-life. To overcome these limitations, we investigated the effect of a low-dose combination of a GLP-1 analogue and FGF21 on atherosclerosis-related molecular pathways.
Methods
C57BL/6J mice were fed a high-fat diet for 30 weeks followed by an atherogenic diet for 10 weeks and were divided into four groups: control (saline), liraglutide (0.3 mg/kg/day), FGF21 (5 mg/kg/day), and low-dose combination treatment with liraglutide (0.1 mg/kg/day) and FGF21 (2.5 mg/kg/day) (n=6/group) for 6 weeks. The effects of each treatment on various atherogenesis-related pathways were assessed.
Results
Liraglutide, FGF21, and their low-dose combination significantly reduced atheromatous plaque in aorta, decreased weight, glucose, and leptin levels, and increased adiponectin levels. The combination treatment upregulated the hepatic uncoupling protein-1 (UCP1) and Akt1 mRNAs compared with controls. Matric mentalloproteinase-9 (MMP-9), monocyte chemoattractant protein-1 (MCP-1), and intercellular adhesion molecule-1 (ICAM-1) were downregulated and phosphorylated Akt (p-Akt) and phosphorylated extracellular signal-regulated kinase (p-ERK) were upregulated in liver of the liraglutide-alone and combination-treatment groups. The combination therapy also significantly decreased the proliferation of vascular smooth muscle cells. Caspase-3 was increased, whereas MMP-9, ICAM-1, p-Akt, and p-ERK1/2 were downregulated in the liraglutide-alone and combination-treatment groups.
Diabetes mellitus is a metabolic disorder that is associated with an increased risk of several comorbidities, including cardiovascular disease (CVD). Atherosclerotic CVD, such as myocardial infarction or stroke, is the main cause of death (accounting for almost 80% of the death) in patients with type 2 diabetes [1]. Atherosclerosis is accompanied by vascular endothelial dysfunction, leading to hardening of the arteries and arterial occlusion, which is a fundamental process underlying CVD. The development of atherosclerosis is complex and influenced by glucose and lipid metabolism, inflammation, and oxidative stress [2,3].
The glucagon-like peptide 1 (GLP-1) receptor has been a treatment target for diabetes. GLP-1 analogues improve insulin sensitivity in the liver and adipose tissue, reducing macrophage infiltration and inhibiting inflammation [4–7]. GLP-1 has multiple biological functions related to the cardiovascular (CV) system [8]. The CV safety and benefits of GLP-1 analogues were reported in recent CV outcome trials [9–13]. However, these drugs, particularly at high-dose, exhibit side effects, such as nausea, vomiting, and other types of gastrointestinal discomfort [14].
The fibroblast growth factor 21 (FGF21), which is a member of the FGF superfamily, is expressed in several tissues, including the liver [15]. FGF21 has emerged as an important regulator of glucose and lipid metabolism [16,17]. Recent studies reported that FGF21 decreased hepatic steatosis, blood glucose, insulin, glucagon, and body weight, and improved insulin sensitivity in rodent models [18,19]. Treatment with FGF21 decreased the development and progression of atherosclerosis in an animal model [20]. Furthermore, FGF21 therapy decreased the concentrations of low-density lipoprotein cholesterol and alleviated insulin resistance in animal models of obesity [18,21]. However, the FGF21 protein has unfavorable pharmacokinetic properties, such as susceptibility to in vivo proteolytic degradation and a short half-life (<2 hours) [22].
To avoid the side effects of liraglutide and to overcome the shortcomings of FGF21, a low-dose combination therapy with a GLP-1 analogue and FGF21 might be a preferred strategy to prevent the cardiometabolic risk associated with diabetes, by possibly lowering glucose levels and reducing body fat and CV risk. Thus, we aimed to estimate the ability of a low-dose combination of liraglutide, which is a GLP-1 analogue, and FGF21 to mitigate the vascular complications of diabetes. Thus, the effect of the GLP-1 analogue/FGF21 combination therapy on vascular cell proliferation, inflammation, and atherosclerosis was explored in a high-fat diet-induced obese mouse model and in vascular smooth muscle cells (VSMCs). Finally, the mechanisms underlying the effects of the combination therapy were investigated using molecular experiments.
Six-to-eight-week-old C57BL/6J male mice were obtained from Jackson Laboratories (Bar Harbor, ME, USA). All mice received a Western diet (40% fat and 43% carbohydrate) for 30 weeks and an atherogenic diet (4% cholesterol, 1% cholic acid, and 0.5% 2-thiouracil) for another 10 weeks, and were divided into four treatment groups: control (saline); liraglutide (0.3 mg/kg/day), as a GLP-1 analogue; FGF21 (LY 2405319; donated by Eli Lilly and Company, Indianapolis, IN, USA; 5 mg/kg/day); and low-dose combination treatment with liraglutide (0.1 mg/kg/day) and FGF21 (2.5 mg/kg/day) (n=6/group) for 6 weeks. The investigational products were administered intraperitoneally. Mice were housed in a temperature-controlled room and maintained under a 12/12-hour light/dark cycle. Mice were sacrificed at 55 weeks of age.
This study was approved by the Institutional Animal Care Committee, Seoul National University Bundang Hospital (SNUBH) (IACUC No. BA1710-233/083-01). The animal experiments were performed in compliance with the Guide for Experimental Animal Research of the Laboratory for Experimental Animal Research, Clinical Research Institute, SNUBH, South Korea, and conformed to the provisions of the Declaration of Helsinki (2013).
To measure the area of atherosclerotic lesions, whole aortas prepared using the en face method were stained with Oil-Red-O solution [23]. After perfusion fixation of the euthanized mice, the whole aorta was dissected out, opened longitudinally from the heart to the bifurcation of the iliac arteries, and stained with Oil-Red-O. Each specimen was evaluated in a blinded fashion. Images of the aorta were analyzed using Image-Pro Plus software (Media Cybernetics, Silver Spring, MD, USA), and results are presented as the area of atheromatous plaque lesion calculated as a percentage of the whole aortic area.
During the treatment period, body weight was measured twice a week. Food and water intake were also measured every week throughout the treatment period. Fasting blood glucose concentrations were measured twice a week using a glucometer (Accu-Chek Inform II, Roche, Mannheim, Germany). Blood samples were collected from mice after 8 hours of fasting at the end of the treatment period. The levels of insulin, glucagon, leptin, ghrelin, and resistin were measured using a Multiplex Assay Kit (MMHMAG-44K, Lot. 3200934, Millipore, Billerica, MA, USA). Adiponectin concentrations were measured using enzyme-linked immunosorbent assay kits (MRP300, R&D system, Minneapolis, MN, USA) according to the manufacturer’s instructions [24].
Rat aortic smooth muscle cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) with 10% fetal bovine serum (HyClone, Logan, UT, USA) in a humidified atmosphere of 5% CO2 and 95% air at 37°C. The medium was replaced every 2 days. Prior to the experiments, the cells were plated in 96-well plates at a density of approximately 1.5×104 cells per well for 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay, or in 6-well plates at 5×106 cells per well for all other assays. For the experiments, the cells were incubated with the agents for 24 hours at 37°C. In each experiment, treatments were performed in triplicate.
Cells were cultured to 80% to 90% confluence and then starved in serum-free DMEM for 24 hours. The medium was replaced with fresh medium containing different concentrations of liraglutide and FGF21 in the presence of the platelet-derived growth factor (PDGF; 10 ng/mL). PDGF is a growth factor that is commonly used to induce the proliferation of vascular cells [25]. Cell viability was analyzed using the MTS cell proliferation assay (CellTiter 96 AQueous Cell Proliferation Assay Kit, Promega Corp., Madison, WI, USA), according to the manufacturer’s protocol. Briefly, the cells were cultured for 24 hours and 20 μL/well of the MTS solution was added to the samples in 100 μL of culture medium. The cells were subsequently incubated at 37°C for 4 hours and the absorbance was measured at 490 nm using a microplate reader.
Cells were grown to 90% confluence and then subjected to scratching using a sterile pipette tip. The medium was replaced with fresh medium containing different concentrations of liraglutide and FGF21 in the presence of PDGF (10 ng/mL). The scratch wound was allowed to heal for 24 hours in the presence of the indicated chemicals. A phase-contrast microscope (Optika, Ponteranica, Italy) captured images of each sample at 0 and 24 hours, and the ability of VSMCs to migrate was evaluated by measuring the area of the scratch wound at both time points, using Image J software version 1.29x (National Institutes of Health, Bethesda, MD, USA).
To determine the rate of apoptosis, the cells were incubated in culture medium containing liraglutide and FGF21 for 24 hours and stained with Annexin V/fluorescein isothiocyanate (FITC), according to the manufacturer’s protocol (Molecular Probes, Thermo Fisher Scientific, Inc., Waltham, MA, USA). Approximately 1×105 cells were harvested and washed with phosphate-buffered saline. Cells were then resuspended in 100 μL of Annexin V binding buffer (10 mM HEPES, 140 mM NaCl, and 2.5 mM CaCl2; pH 7.4), incubated with 5 μL of Annexin V/FITC for 15 minutes at room temperature, and counterstained with propidium iodide (PI; final concentration, 1 μg/mL) for 10 minutes at room temperature. After the incu-bation period, the cells were diluted with 190 μL of Annexin V binding buffer and analyzed by flow cytometry using a Becton Dickinson FACScan flow cytometer with the Cell Quest 3.1 software (BD Biosciences, Franklin Lakes, NJ, USA).
Total RNA was extracted from the cells according to the Promega Total RNA Isolation System manual. Reverse transcription quantitative polymerase chain reaction was performed on an ABI Prism 7500 Sequence Detection System (Applied Biosystems Inc., Foster City, CA, USA), according to the manufacturer’s instructions. SYBR Green (Toyobo Corp., Osaka, Japan) was used as a double stranded DNA-specific fluorescent dye. The specific primers used here were as follows: uncoupling protein-1 (UCP1) forward, 5′–ATCGGCCTCTACGACAACGTCAA–3′ and reverse, 5′–ACCAGTTCTGTGCAGTTGACCAG–3′; Akt1 forward, 5′–ACTCATTCCAGACCCACGAC–3′ and reverse, 5′–ATACACATCCTGCCACACGA–3′; and β actin forward, F 5′–CGTTGACATCCGTAAAGACCTC–3′ and reverse, 5′–TAGGAGCCAGGGCAGTAATCT–3′.
Cells (1×106/mL) were resuspended in a lysis buffer (Cell Signaling Technology Inc., Danvers, MA, USA) containing a protease inhibitor cocktail (cOmplete Mini Protease Inhibitor Tablet, Roche Diagnostics, Rotkreuz, Switzerland). The protein concentrations were measured in the supernatant using a Pierce BCA Protein Assay Kit (cat. no. 23225, Thermo Fisher Scientific Inc.). A total of 40 μg of protein was loaded per gel lane, separated via 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and transferred onto nitrocellulose membranes. The membranes were blocked for 2 hours at room temperature with 5% skimmed milk in Tris-buffered saline with Tween 20 (TBST; 20 mM Tris, 500 nM NaCl, and 0.1% Tween 20), followed by incubation with primary antibodies against cleaved caspase-3, matric mentalloproteinase-9 (MMP-9), intercellular adhesion molecule-1 (ICAM-1), monocyte chemoattractant protein-1 (MCP-1), phosphorylated Akt (p-Akt), phosphorylated extracellular signal-regulated kinase (p-ERK), phosphorylated p38 (p-p38), and phosphorylate JNK (p-JNK) (all 1:1,000; Cell Signaling Technology, Boston, MA, USA), and β-actin (1:5,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight at 4°C. After three washes with TBST, the membranes were incubated with secondary horseradish peroxidase conjugated anti immunoglobulin G antibodies (1:5,000; Invitrogen; Thermo Fisher Scientific Inc.) for 2 hours at room temperature and visualized using a Pierce enhanced chemiluminescence substrate (Thermo Fisher Scientific Inc.). Densitometric quantification of the protein bands was achieved using the ImageJ software version 1.29x (National Institutes of Health).
Quantitative data are presented as mean±standard deviation for animal studies and mean±standard error for cell studies. Differences between mean values were compared statistically by two-tailed Student’s t test or one-way analysis of variance followed by Tukey’s post hoc comparison. Statistical analyses were performed using the SPSS Windows software version 22.0 (IBM Corp., Armonk, NY, USA). Significance was set at P<0.05. All experiments were performed at least three times.
Plaque accumulation in the aorta of C57BL/6J male mice is shown in Fig. 1. Quantification showed that the plaque areas were significantly smaller in the groups treated with liraglutide, with FGF21, or their combination than in controls (all P<0.05). Moreover, the low-dose combination therapy of liraglutide (0.1 mg/kg) and FGF21 (2.5 mg/kg) showed significantly lower atheromatous plaque formation in the aorta than either liraglutide alone or FGF21 alone (both P<0.05) (Fig. 1).
Among the animals that were fed a high-fat and atherogenic diet, weight was lower in the liraglutide, FGF21, and low-dose combination of liraglutide and FGF21 groups compared with the control group, starting at 13 days after treatment and with the exception of days 40 and 42 (Fig. 2A). Random glucose concentrations were lower in the liraglutide, FGF21, and low-dose combination of liraglutide and FGF21 groups compared with the control group, starting at 11 days after treatment and with the exception of days 23, 31, and 35 (Fig. 2B). Similarly, food intake was lower in the liraglutide alone and low-dose combination of liraglutide and FGF21 groups than in the FGF21 or control groups starting at 36 days after treatment (Fig. 2C).
Liraglutide, FGF21, and their low-dose combination regimen decreased insulin levels, albeit without statistical significance. Glucagon levels were significantly suppressed only by the liraglutide treatment (Fig. 3). All three treatments increased the levels of circulating adiponectin in these high-fat diet-fed mice. Furthermore, leptin levels were significantly lower in all treatment groups. Finally, the levels of both ghrelin and resistin exhibited decreasing patterns, albeit without statistical significance (Fig. 3).
Both liraglutide alone and its combination with FGF21 downregulated MMP-9, MCP-1, and ICAM-1 in the mouse liver compared with the control group (Fig. 4A). To investigate whether UCP1 and Akt1 were involved in the regulation of glucose metabolism in the combination treatment, the hepatic expression levels of UCP1 and Akt1 were tested. As shown in Fig. 4D, the mRNA expression of UCP1 and Akt1 was significantly increased in animals treated with a low-dose combination of liraglutide and FGF21 compared with the controls or either liraglutide-alone- or FGF21-alone-treated mice. Therefore, the liraglutide and FGF21 combination treatment exerted a synergistic effect on the expression of these molecules.
To identify the signaling pathways involved in the effects of the liraglutide and FGF21 combination treatment, we examined the phosphorylation of Akt and ERK by Western blotting. In the liver and visceral fat of obese mice, p-Akt and p-ERK were upregulated in the liraglutide-treated and combination groups compared with the control group (Fig. 4B, C). Moreover, in the liver, the combination treatment exerted a partial additive effect.
The PDGF-induced proliferative effects were significantly reversed by liraglutide concentrations above 50 μM and by the combined treatment with concentrations above 50 μM liraglutide and FGF21 (Fig. 5A). Subsequent experiments were performed using toxic cellular concentrations (50 μM liraglutide alone or 10 μM liraglutide combined with 50 nM FGF21). We focused on further defining the therapeutic combinational potential of liraglutide and FGF21.
A wound-healing migration assay was performed to determine the effects of liraglutide alone or the liraglutide and FGF21 combination treatment on PDGF-induced VSMC migration. VSMC motility was significantly increased after a 24-hour treatment with PDGF compared with both the liraglutide-alone and the combination groups (Fig. 5B).
To investigate the possible anti-apoptotic effects of the liraglutide and FGF21 combination therapy, a flow cytometry assay was performed using VSMCs. After 24 hours of treatment with the drugs, approximately 19.03% and 14.43% of apoptotic cells were detected in the liraglutide-alone and the combination groups, respectively. In addition, early apoptosis was dramatically increased in cells treated with liraglutide alone or the liraglutide and FGF21 combination compared with PDGF-stimulated cells. Therefore, the proportion of apoptotic cells was higher upon treatment with liraglutide alone and the liraglutide and FGF21 combination compared with PDGF treatment (Fig. 6A). The activation of caspase-3 is marked by the cleavage of procaspase-3 and is the executioner of apoptosis. We examined the levels of cleaved caspase-3 by Western blotting. Apoptosis was effectively induced in VSMCs treated with liraglutide and the liraglutide and FGF21 combination compared with PDGF-stimulated cells (Fig. 6B).
The expression of MMP-9 and ICAM-1 was significantly suppressed in VSMCs after treatment with both liraglutide alone and the liraglutide and FGF21 combination compared with PDGF treatment (Fig. 7A). As shown in Fig. 7B, the treatment with liraglutide, either alone or in combination with FGF21, significantly suppressed the PDGF-induced phosphorylation of Akt and ERK in VSMCs.
In the Western blot analysis, there was significantly increased expression of p-p38 in the liraglutide alone treatment in the pancreas and in the liraglutide alone and liraglutide and FGF21 combination treatment in the pancreas and white adipose tissue (WAT) (Supplemental Fig. S1). There was a significant decrease in the expression of p-JNK by FGF21 alone treatment in the liver. Otherwise, there were no changes in the expression levels of these two proteins in the liver, pancreas, and WAT by treatments with liraglutide alone, FGF21 alone, or a low-dose combination of both.
Here, a low-dose combination of liraglutide and FGF21 reduced atheromatous plaque area in aorta and decreased body weight and blood glucose concentrations in high-fat diet-induced obese mice compared with controls and caused a concomitant upregulation of the UCP1 and Akt1 mRNA. The low-dose combination of liraglutide and FGF21 suppressed the proliferation of VSMCs, accompanied by suppression of MMP-9 and ICAM-1, thereby reducing the development of atherosclerosis. Furthermore, treatment with this combination therapy downregulated the inflammatory markers MMP-9 and MCP-1, as well as the adhesion molecule ICAM-1. Our in vitro and in vivo experiments showed that the anti-inflammatory and anti-atherosclerotic effects of the GLP-1 analogue and FGF21 combination therapy occurred via the Akt and ERK pathways. To the best of our knowledge, this is the first study that has demonstrated that the combination of a GLP-1 analogue and FGF21 is effective against the vascular complications of diabetes.
A recent study using male C57BL/6 mice reported that the UCP1 protein is expressed in the liver as well as in WAT [26]. In that study, a ketogenic diet upregulated UCP1 in the liver. Another study using Sprague Dawley rats also reported that liraglutide injection increased the expression of the UCP1 gene and UCP1 protein in the liver [27]. UCP1 is a mitochondrial protein that is involved in inflammatory cytokine signaling pathways and is implicated in liver function. Therefore, UCP1 is of great significance for application as a reasonable indicator of metabolic health. Moreover, UCP1 activation in brown adipose tissue is essential for the maintenance of the core body temperature, and is associated with liver steatosis and oxidative stress [28,29]. Moreover, Akt activation results in marked alterations in glucose and lipid metabolism [30]. Here, we showed that the low-dose combination of a GLP-1 analogue and FGF21 exerted body-weight-reducing effects, decreased fasting glucose concentration, and upregulated the UCP1 and Akt1 mRNAs, suggesting that this treatment reduces obesity and improves insulin sensitivity by inducing UCP1 and Akt gene expression. The elevated adiponectin levels and the enhancement in mitochondrial function induced by the combination therapy may play an intrinsic role in the upregulation of UCP1 and Akt1 in the liver [27,31]. There is no clear answer to why treatments with liraglutide alone or FGF21 alone treatment did not increase UCP1 expression. We speculate that these two molecules might act synergistically. A previous study showed that UCP1-dependent thermogenesis was not required for FGF21 to improve glycemic control or to reduce the levels of circulating cholesterol or free fatty acids [32]. Thus, some metabolic actions of FGF21 are not associated with UCP1.
In the current study, the food intake was lower in the liraglutide-alone or liraglutide and FGF21 combination groups than in the control group. The body-weight-reducing effects of GLP-1/FGF21 treatment could be also due to increased energy expenditure, leading to a negative energy balance. Energy expenditure has different elements, such as resting metabolic rate, physical activity, and thermogenesis. It also depends on the oxidation of substrates. It was reported that GLP-1 administration increased whole-body oxygen consumption in rats, an index of energy expenditure and body temperature by 0.3°C [33]. In another animal study, GLP-1 administration increased thermogenesis in brown adipose tissue [34]. Unfortunately, we did not measure body temperature in the present study. Taken together, these preclinical findings indicate that GLP-1 analogues might influence the central regulation of energy balance by affecting dietary intake, energy expenditure, and thermogenesis in particular.
Here, liraglutide, FGF21, and their combination increased adiponectin levels and decreased leptin levels. Decreased serum levels of adiponectin have been suggested as a mechanism underlying obesity-induced insulin resistance [35]. Adiponectin possesses potent anti-inflammatory and anti-atherosclerotic activities via its multiple actions in the vascular system [36]. Leptin is a crucial hormone that is responsible for the endocrine control of energy homeostasis [37]. The development of resistance to leptin is a hallmark of obesity [38]. The favorable changes in adiponectin and leptin afforded by liraglutide alone or the combination treatment with liraglutide and FGF21 suggest their systemic effects on the cardiometabolic system and energy metabolism.
Our group showed that a synthetic GLP-1 molecule together with exenatide, another GLP-1 analogue, attenuated the PDGF-induced proliferation of VSMCs [39]. Another study showed that GLP-1 treatment effectively attenuated the proliferation of VSMCs induced by angiotensin II [40]. Proliferation and migration of PDGF-induced VSMCs accompany the formation of vascular injury, which leads to the development of atherosclerosis [41]. Apoptosis is also important for the control of excessive cell proliferation and elimination of harmful cells, being regulated both positively and negatively by growth factors, including IGF-1 and PDGF [42,43]. A study reported that liraglutide inhibits VSMC proliferation by promoting cell-cycle arrest via the activation of AMPK [40]. The imbalance between VSMC proliferation and apoptosis is related to neointimal formation, indicating high proliferation/apoptosis ratio in VSMCs in the arterial wall after injury or inflammatory stimuli [44]. In the present study, we found that liraglutide alone (50 and 100 μM) or in combination with FGF21 inhibited the proliferation of VSMCs in response to PDGF. The liraglutide-alone and liraglutide and FGF21 combination treatments increased the proportion of apoptotic cells and the levels of caspase-3. However, the FGF21 alone treatment (25 and 50 nM) showed a slight decrease in the proliferation of VSMCs but this lacked statistical significance. This might have been because of the use of low-dose FGF21. From a different context, the vascular endothelial cells lining the atheromatous region and apoptosis of vascular endothelial cells are critical in maintaining the stability of atheromatous plaques [45]. Apoptosis of VSMCs directly comprising atheromatous plaques might confer instability, but in contrast, other reports suggest that apoptosis of VSMCs contributes to the reduced development of atheromatous plaques [39,46]. Taken together, our findings suggest that the combination of liraglutide and FGF21 suppresses the proliferation of VSMCs more efficiently by inducing apoptosis.
Atherosclerosis is an inflammatory disease and a major cause of the upregulation of adhesion molecules for leukocytes in VSMCs via altered phenotypic expression at the injured sites [47]. MMPs are proteolytic enzymes that act on a variety of connective tissue proteins, and increasing evidence supports their involvement in the development of atherosclerotic plaques [48]. MCP-1 activates monocytes/macrophages at the site of tissue injury and regulates adhesion molecules [49]. The expression of MMP-9 and ICAM-1 is increased in VSMCs during the development of atherosclerosis [50,51]. In addition, monocytes expressing MCPs affect the growth of cells within atherosclerotic lesions [52]. Importantly, GLP-1-based treatment yielded beneficial anti-inflammatory effects in the liver and vein endothelial cells, and the use of FGF21 prevented the inflammation and oxidative stress associated with atherosclerosis in vivo [53,54]. Here, in vivo experiments showed that the combination of liraglutide and FGF21 downregulated MMP-9, MCP-1, and ICAM-1 and increased the plasma levels of adiponectin. These data support the notion that decreased inflammation is closely related to the inhibition of atherosclerosis in diabetes. Thus, liraglutide and FGF21 may exert a synergistic effect in the prevention of diabetes-associated atherosclerosis, with a possibly higher anti-inflammatory potential than that observed for GLP-1, FGF21, and other monotherapies.
In our experiment, the most noteworthy effect of the GLP-1 analogue was its anti-inflammatory ability. FGF21 induced sizable improvements in hepatic steatosis [55]. Consequently, the GLP-1 analogue and FGF21 combination therapy may be more effective at promoting insulin sensitivity in the liver, compared with the GLP-1 analogue alone. In addition, the combination treatment significantly reduced PDGF-stimulated Akt and ERK1/2 phosphorylation in VSMCs, suggesting its therapeutic applicability in the prevention of atherosclerosis. Our in vivo experiments explored the molecular events downstream of insulin signaling and showed that the liraglutide and FGF21 combination significantly increased Akt and ERK1/2 phosphorylation in the liver compared with high-fat-fed control mice. Because Akt and ERK1/2 are signaling molecules that are involved in the pathophysiology of vascular complications, these results support the beneficial effect of the two agents on vasculature in a synergistic fashion.
Gastrointestinal side effects are common in high-dose GLP-1 analogue therapy [14]. In addition, the FGF21 protein has several limitations as a therapeutic agent, including a short half-life [22]. To reduce the side effects induced by liraglutide treatment and overcome the shortcomings of FGF21, a low-dose combination of the two agents might be an ideal strategy from a safety perspective.
In conclusion, the present study expands our understanding of the effects elicited by the combination of a GLP-1 analogue with FGF21 and provides new insights regarding an ideal therapeutic option for preventing the development of atherosclerosis in patients with diabetes. We showed an additive or partial synergistic effect of the liraglutide and FGF21 combination compared with the control or the single drugs in several in vivo experiments. Thus, FGF21 might be a suitable agent to enhance the effect of GLP-1 analogues. Taken together, our results indicate that, by acting on the Akt and ERK1/2 pathways, the combined treatment with a GLP-1 analogue and FGF21 protects against inflammation and atherosclerosis. These results suggest that the GLP-1 analogue and FGF21 combination is of potential clinical relevance in the treatment of vascular complications related to diabetes.
Supplementary Information
Supplemental Fig. S1.
Effect of liraglutide (0.3 mg/kg), fibroblast growth factor 21 (FGF21; 5 mg/kg), and low-dose combination of liraglutide (0.1 mg/kg) plus FGF21 (2.5 mg/kg) treatment on phosphorylated p38 (p-p38) and phosphorylated JNK (p-JNK) in liver. aP<0.05 vs. control, liraglutide, and low-dose combination of liraglutide and FGF21.
ACKNOWLEDGMENTS
This study was supported by the National Research Fund, the Ministry of Health and Welfare, Republic of Korea (2017R1D1A1B03035165) and Seoul National University Bundang Hospital. The funders had no role in study design, data collection and analysis, or preparation of the manuscript. FGF21 compound (LY 2405319) was donated from Eli Lilly (Indianapolis, IN, USA).
Notes
AUTHOR CONTRIBUTIONS
Conception or design: J.H.K., G.Y.L., H.J.M., H.K., J.H.B., K.M.K., S.L. Acquisition, analysis, or interpretation of data: J.H.K., G.Y.L., H.J.M., H.K., J.H.B., K.M.K., S.L. Drafting the work or revising: J.H.K., S.L. Final approval of the manuscript: J.H.K., G.Y.L., H.J.M., H.K., J.H.B., K.M.K., S.L.
REFERENCES
1. Martin-Timon I, Sevillano-Collantes C, Segura-Galindo A, Del Canizo-Gomez FJ. Type 2 diabetes and cardiovascular disease: have all risk factors the same strength? World J Diabetes. 2014; 5:444–70.

2. Abdul-Ghani M, DeFronzo RA, Del Prato S, Chilton R, Singh R, Ryder REJ. Cardiovascular disease and type 2 diabetes: has the dawn of a new era arrived? Diabetes Care. 2017; 40:813–20.

3. Aronson D, Bloomgarden Z, Rayfield EJ. Potential mechanisms promoting restenosis in diabetic patients. J Am Coll Cardiol. 1996; 27:528–35.

4. Ben-Shlomo S, Zvibel I, Shnell M, Shlomai A, Chepurko E, Halpern Z, et al. Glucagon-like peptide-1 reduces hepatic lipogenesis via activation of AMP-activated protein kinase. J Hepatol. 2011; 54:1214–23.

5. Chai W, Dong Z, Wang N, Wang W, Tao L, Cao W, et al. Glucagon-like peptide 1 recruits microvasculature and increases glucose use in muscle via a nitric oxide-dependent mechanism. Diabetes. 2012; 61:888–96.

6. Gao H, Wang X, Zhang Z, Yang Y, Yang J, Li X, et al. GLP-1 amplifies insulin signaling by up-regulation of IRbeta, IRS-1 and Glut4 in 3T3-L1 adipocytes. Endocrine. 2007; 32:90–5.
7. Lee YS, Park MS, Choung JS, Kim SS, Oh HH, Choi CS, et al. Glucagon-like peptide-1 inhibits adipose tissue macrophage infiltration and inflammation in an obese mouse model of diabetes. Diabetologia. 2012; 55:2456–68.

8. Lim S, Kim KM, Nauck MA. Glucagon-like peptide-1 receptor agonists and cardiovascular events: class effects versus individual patterns. Trends Endocrinol Metab. 2018; 29:238–48.

9. Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017; 377:1228–39.

10. Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016; 375:1834–44.

11. Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016; 375:311–22.

12. Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Kober LV, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015; 373:2247–57.

13. Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomized placebo-controlled trial. Lancet. 2019; 394:121–30.
14. Ard J, Cannon A, Lewis CE, Lofton H, Vang Skjoth T, Stevenin B, et al. Efficacy and safety of liraglutide 3.0 mg for weight management are similar across races: subgroup analysis across the SCALE and phase II randomized trials. Diabetes Obes Metab. 2016; 18:430–5.

16. Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, et al. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005; 115:1627–35.

17. Nishimura T, Nakatake Y, Konishi M, Itoh N. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim Biophys Acta. 2000; 1492:203–6.

18. Kharitonenkov A, Wroblewski VJ, Koester A, Chen YF, Clutinger CK, Tigno XT, et al. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology. 2007; 148:774–81.

19. Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G, et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes. 2009; 58:250–9.

20. Lin Z, Pan X, Wu F, Ye D, Zhang Y, Wang Y, et al. Fibroblast growth factor 21 prevents atherosclerosis by suppression of hepatic sterol regulatory element-binding protein-2 and induction of adiponectin in mice. Circulation. 2015; 131:1861–71.

21. Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab. 2007; 5:415–25.
22. So WY, Leung PS. Fibroblast growth factor 21 as an emerging therapeutic target for type 2 diabetes mellitus. Med Res Rev. 2016; 36:672–704.

23. Han JH, Oh TJ, Lee G, Maeng HJ, Lee DH, Kim KM, et al. The beneficial effects of empagliflozin, an SGLT2 inhibitor, on atherosclerosis in ApoE (−/−) mice fed a western diet. Diabetologia. 2017; 60:364–76.

24. Neuhoff S, Moers J, Rieks M, Grunwald T, Jensen A, Dermietzel R, et al. Proliferation, differentiation, and cytokine secretion of human umbilical cord blood-derived mononuclear cells in vitro. Exp Hematol. 2007; 35:1119–31.

25. Hur KY, Seo HJ, Kang ES, Kim SH, Song S, Kim EH, et al. Therapeutic effect of magnesium lithospermate B on neointimal formation after balloon-induced vascular injury. Eur J Pharmacol. 2008; 586:226–33.

26. Chapnik N, Genzer Y, Froy O. Relationship between FGF21 and UCP1 levels under time-restricted feeding and high-fat diet. J Nutr Biochem. 2017; 40:116–21.

27. Decara J, Arrabal S, Beiroa D, Rivera P, Vargas A, Serrano A, et al. Antiobesity efficacy of GLP-1 receptor agonist liraglutide is associated with peripheral tissue-specific modulation of lipid metabolic regulators. Biofactors. 2016; 42:600–11.

28. Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004; 84:277–359.

29. Liu P, Yang J, Chen ZY, Zhang P, Shi GJ. Mitochondrial protein UCP1 mediates liver injury induced by LPS through EKR signaling pathway. Eur Rev Med Pharmacol Sci. 2017; 21:3674–9.
30. Ono H, Shimano H, Katagiri H, Yahagi N, Sakoda H, Onishi Y, et al. Hepatic Akt activation induces marked hypoglycemia, hepatomegaly, and hypertriglyceridemia with sterol regulatory element binding protein involvement. Diabetes. 2003; 52:2905–13.

31. Zhou J, Poudel A, Chandramani-Shivalingappa P, Xu B, Welchko R, Li L. Liraglutide induces beige fat development and promotes mitochondrial function in diet induced obesity mice partially through AMPK-SIRT-1-PGC1-α cell signaling pathway. Endocrine. 2019; 64:271–83.

32. Samms RJ, Smith DP, Cheng CC, Antonellis PP, Perfield JW 2nd, Kharitonenkov A, et al. Discrete aspects of FGF21 in vivo pharmacology do not require UCP1. Cell Rep. 2015; 11:991–9.
33. Osaka T, Endo M, Yamakawa M, Inoue S. Energy expenditure by intravenous administration of glucagon-like peptide-1 mediated by the lower brainstem and sympathoadrenal system. Peptides. 2005; 26:1623–31.

34. Lockie SH, Heppner KM, Chaudhary N, Chabenne JR, Morgan DA, Veyrat-Durebex C, et al. Direct control of brown adipose tissue thermogenesis by central nervous system glucagon-like peptide-1 receptor signaling. Diabetes. 2012; 61:2753–62.

35. Achari AE, Jain SK. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int J Mol Sci. 2017; 18:1321.

36. Hui X, Lam KS, Vanhoutte PM, Xu A. Adiponectin and cardiovascular health: an update. Br J Pharmacol. 2012; 165:574–90.

37. Cui H, Lopez M, Rahmouni K. The cellular and molecular bases of leptin and ghrelin resistance in obesity. Nat Rev Endocrinol. 2017; 13:338–51.

38. Frederich RC, Hamann A, Anderson S, Lollmann B, Lowell BB, Flier JS. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat Med. 1995; 1:1311–4.

39. Lim S, Lee GY, Park HS, Lee DH, Oh TJ, Kim KM, et al. Attenuation of carotid neointimal formation after direct delivery of a recombinant adenovirus expressing glucagon-like peptide-1 in diabetic rats. Cardiovasc Res. 2017; 113:183–94.

40. Jojima T, Uchida K, Akimoto K, Tomotsune T, Yanagi K, Iijima T, et al. Liraglutide, a GLP-1 receptor agonist, inhibits vascular smooth muscle cell proliferation by enhancing AMP-activated protein kinase and cell cycle regulation, and delays atherosclerosis in ApoE deficient mice. Atherosclerosis. 2017; 261:44–51.

41. Jawien A, Bowen-Pope DF, Lindner V, Schwartz SM, Clowes AW. Platelet-derived growth factor promotes smooth muscle migration and intimal thickening in a rat model of balloon angioplasty. J Clin Invest. 1992; 89:507–11.

42. Bennett MR, Evan GI, Schwartz SM. Apoptosis of human vascular smooth muscle cells derived from normal vessels and coronary atherosclerotic plaques. J Clin Invest. 1995; 95:2266–74.

43. Kardas G, Daszynska-Kardas A, Marynowski M, Brzakalska O, Kuna P, Panek M. Role of platelet-derived growth factor (PDGF) in asthma as an immunoregulatory factor mediating airway remodeling and possible pharmacological target. Front Pharmacol. 2020; 11:47.

44. Perlman H, Sata M, Krasinski K, Dorai T, Buttyan R, Walsh K. Adenovirus-encoded hammerhead ribozyme to Bcl-2 inhibits neointimal hyperplasia and induces vascular smooth muscle cell apoptosis. Cardiovasc Res. 2000; 45:570–8.

45. Clarke MC, Figg N, Maguire JJ, Davenport AP, Goddard M, Littlewood TD, et al. Apoptosis of vascular smooth muscle cells induces features of plaque vulnerability in atherosclerosis. Nat Med. 2006; 12:1075–80.

46. Lim S, Lee KS, Lee JE, Park HS, Kim KM, Moon JH, et al. Effect of a new PPAR-gamma agonist, lobeglitazone, on neointimal formation after balloon injury in rats and the development of atherosclerosis. Atherosclerosis. 2015; 243:107–19.

47. Rolfe BE, Muddiman JD, Smith NJ, Campbell GR, Campbell JH. ICAM-1 expression by vascular smooth muscle cells is phenotype-dependent. Atherosclerosis. 2000; 149:99–110.

48. Newby AC. Dual role of matrix metalloproteinases (matrixins) in intimal thickening and atherosclerotic plaque rupture. Physiol Rev. 2005; 85:1–31.

49. Jiang Y, Beller DI, Frendl G, Graves DT. Monocyte chemoattractant protein-1 regulates adhesion molecule expression and cytokine production in human monocytes. J Immunol. 1992; 148:2423–8.
51. Senior RM, Griffin GL, Fliszar CJ, Shapiro SD, Goldberg GI, Welgus HG. Human 92- and 72-kilodalton type IV collagenases are elastases. J Biol Chem. 1991; 266:7870–5.

52. Lin J, Kakkar V, Lu X. Impact of MCP-1 in atherosclerosis. Curr Pharm Des. 2014; 20:4580–8.
53. Lee YS, Jun HS. Anti-inflammatory effects of glp-1-based therapies beyond glucose control. Mediators Inflamm. 2016; 2016:3094642.

54. Jia H, Cheng J, Zhou Q, Peng J, Pan Y, Han H. Fibroblast growth factor 21 attenuates inflammation and oxidative stress in atherosclerotic rat via enhancing the Nrf1-ARE signaling pathway. Int J Clin Exp Pathol. 2018; 11:1308–17.
Fig. 1
Atheroma burden in the aorta of C57BL/6J mice after 6 weeks of treatment with liraglutide (0.3 mg/kg/day), fibroblast growth factor 21 (FGF21; 5 mg/kg/day), and a low-dose combination treatment with liraglutide (0.1 mg/kg/day) and FGF21 (2.5 mg/kg/day) (n=6 in each group). (A) Representative images of atheromata in the aorta stained with Oil-Red-O (scale bar, 0.5 cm). The red color indicates plaque accumulation. (B) The atheromatous plaque area (% of total area) in the aorta. Data are mean±standard deviation. aP<0.05 vs. control; bP<0.05 vs. liraglutide (0.3 mg/kg); cP<0.05 vs. FGF21 (5 mg/kg).
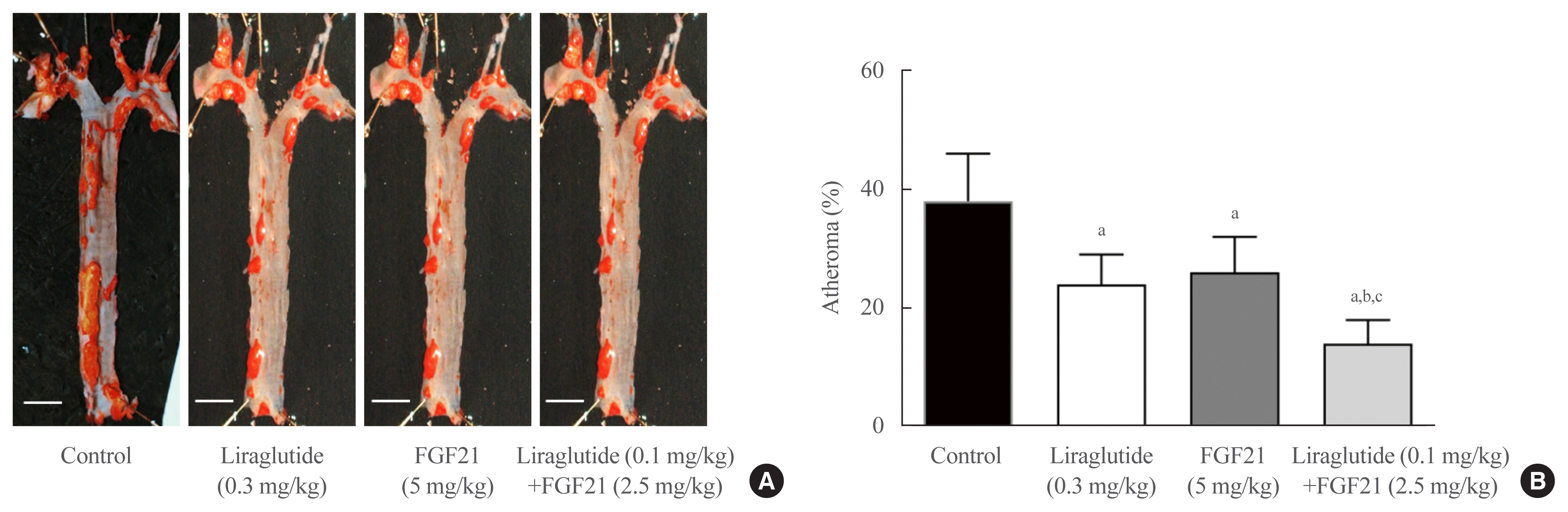
Fig. 2
Effect of a 6-week treatment with liraglutide (0.3 mg/kg/day) alone, fibroblast growth factor 21 (FGF21; 5 mg/kg/day) alone, and liraglutide (0.1 mg/kg/day) plus FGF21 (2.5 mg/kg/day) low-dose combination therapy on (A) body weight, (B) blood glucose concentrations, and (C) food intake. Post hoc analysis by least significant difference t tests of the mean differences between two groups. aControl vs. liraglutide (0.3 mg/kg/day); bControl vs. FGF21 (5 mg/kg/day); cControl vs. low-dose combination treatment with liraglutide (0.1 mg/kg/day) and FGF21 (2.5 mg/kg/day); dFGF21 (5 mg/kg/day) vs. liraglutide (0.3 mg/kg/day); eFGF21 (5 mg/kg/day) vs. low-dose combination treatment with liraglutide (0.1 mg/kg/day) and FGF21 (2.5 mg/kg/day), n=6 in each group, P<0.05 in all cases.
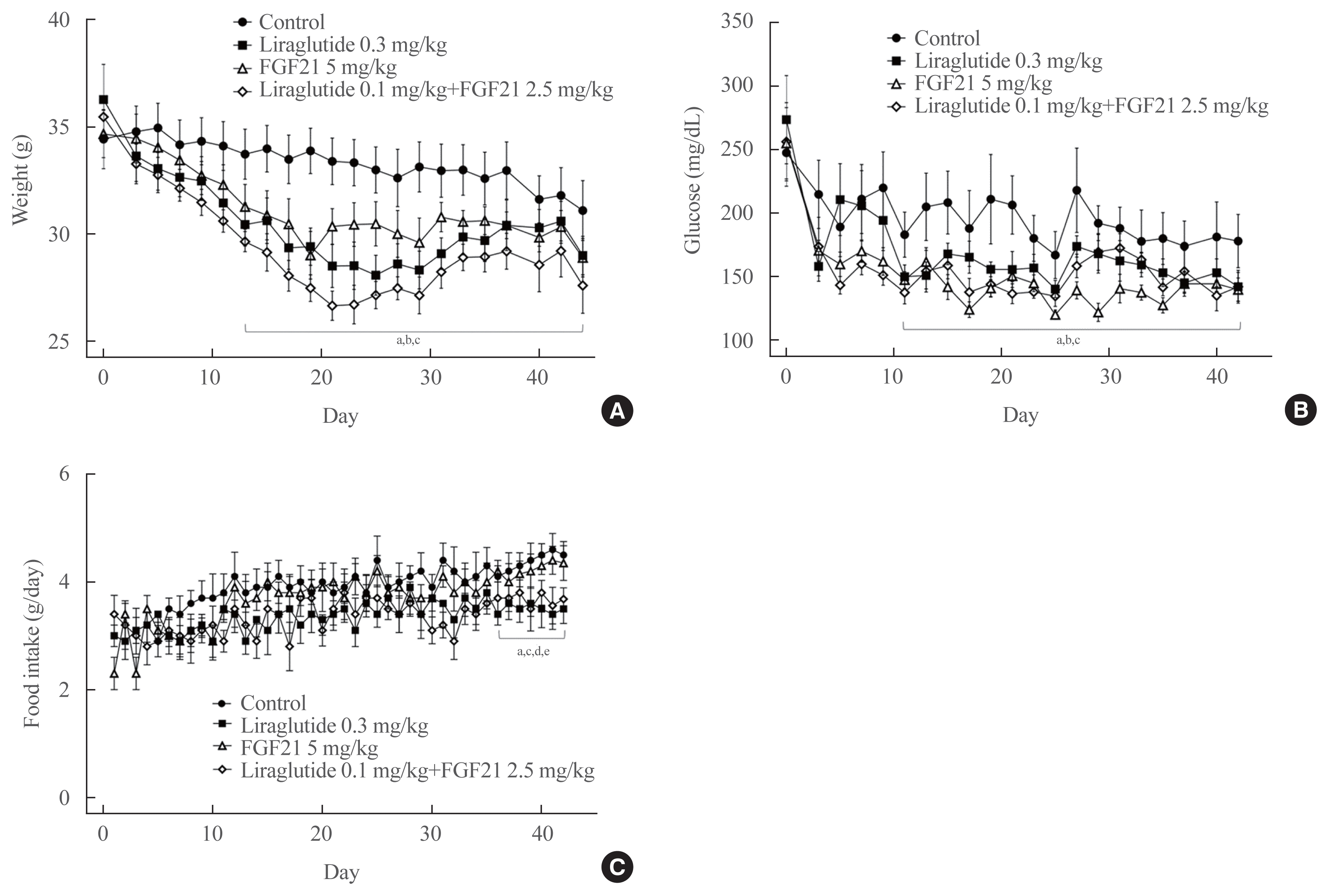
Fig. 3
Effect of liraglutide and fibroblast growth factor 21 (FGF21) on the circulating concentrations of biomarkers. (A) Insulin, (B) glucagon, (C) adiponectin, (D) leptin, (E) ghrelin, and (F) resistin concentrations were measured after 6 weeks of treatment (mean±standard error). aControl vs. liraglutide (0.3 mg/kg/day); bControl vs. FGF21 (5 mg/kg/day); cControl vs. low-dose combination treatment with liraglutide (0.1 mg/kg/day) and FGF21 (2.5 mg/kg/day), n=6 in each group, P<0.05 in all cases.
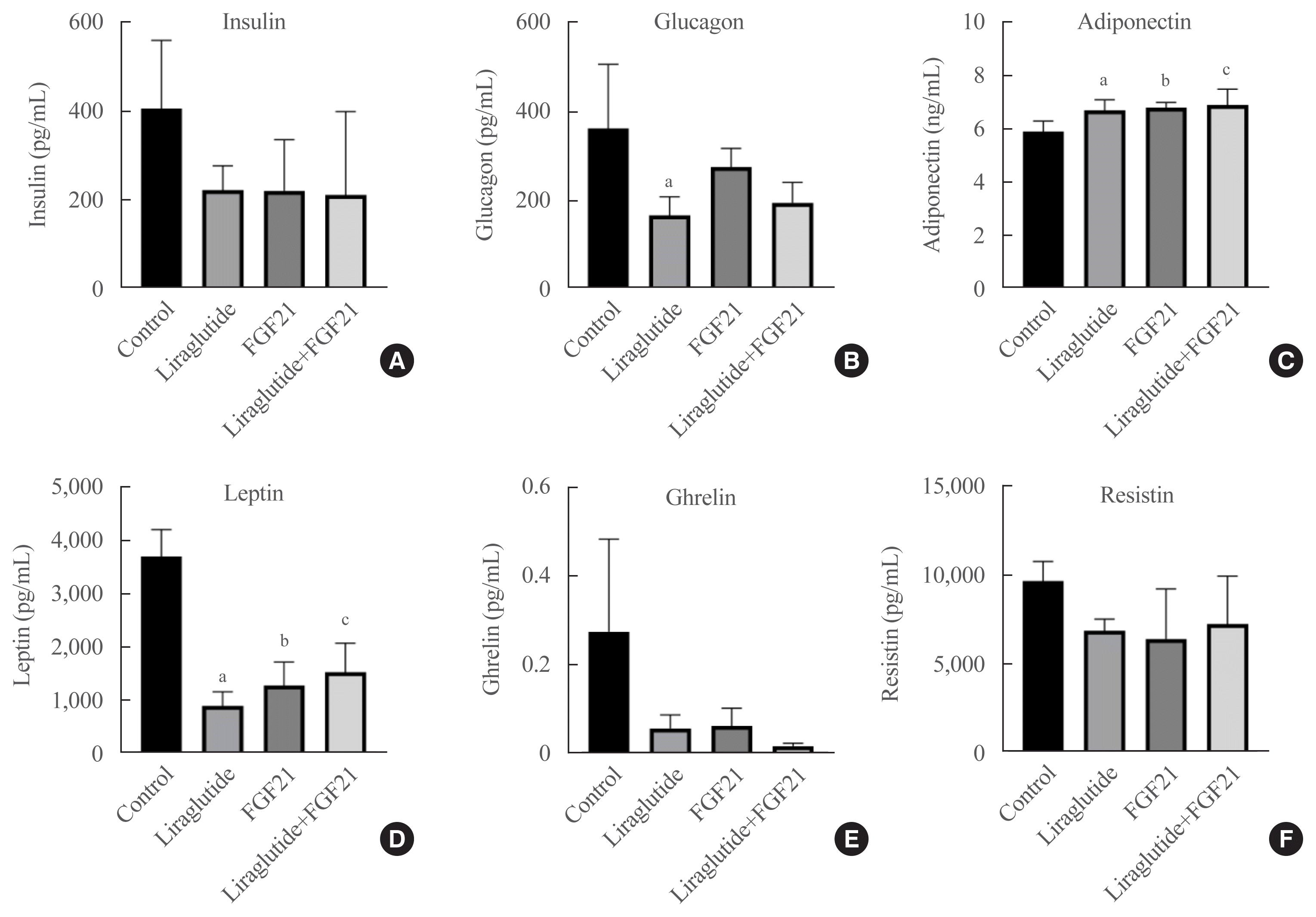
Fig. 4
Effect of liraglutide alone and the liraglutide and fibroblast growth factor 21 (FGF21) combination on inflammation, adhesion molecules, phosphorylated Akt (p-Akt), phosphorylated extracellular signal-regulated kinase (p-ERK), and uncoupling protein-1 (UCP1) in the liver and p-Akt, p-ERK in visceral fat. (A) The expression of matric mentalloproteinase-9 (MMP-9), monocyte chemoattractant protein-1 (MCP-1), and intercellular adhesion molecule-1 (ICAM-1) in mouse liver was decreased after treatment with liraglutide and the liraglutide and FGF21 combination. (B, C) In the liver and visceral fat, treatment with liraglutide or the liraglutide and FGF21 combination increased the levels of p-Akt and p-ERK compared with control animals. (D) Relative expression of the UCP1 and Akt1 mRNA in the mouse liver, as analyzed by quantitative polymerase chain reaction (mean±standard error). aP<0.05.
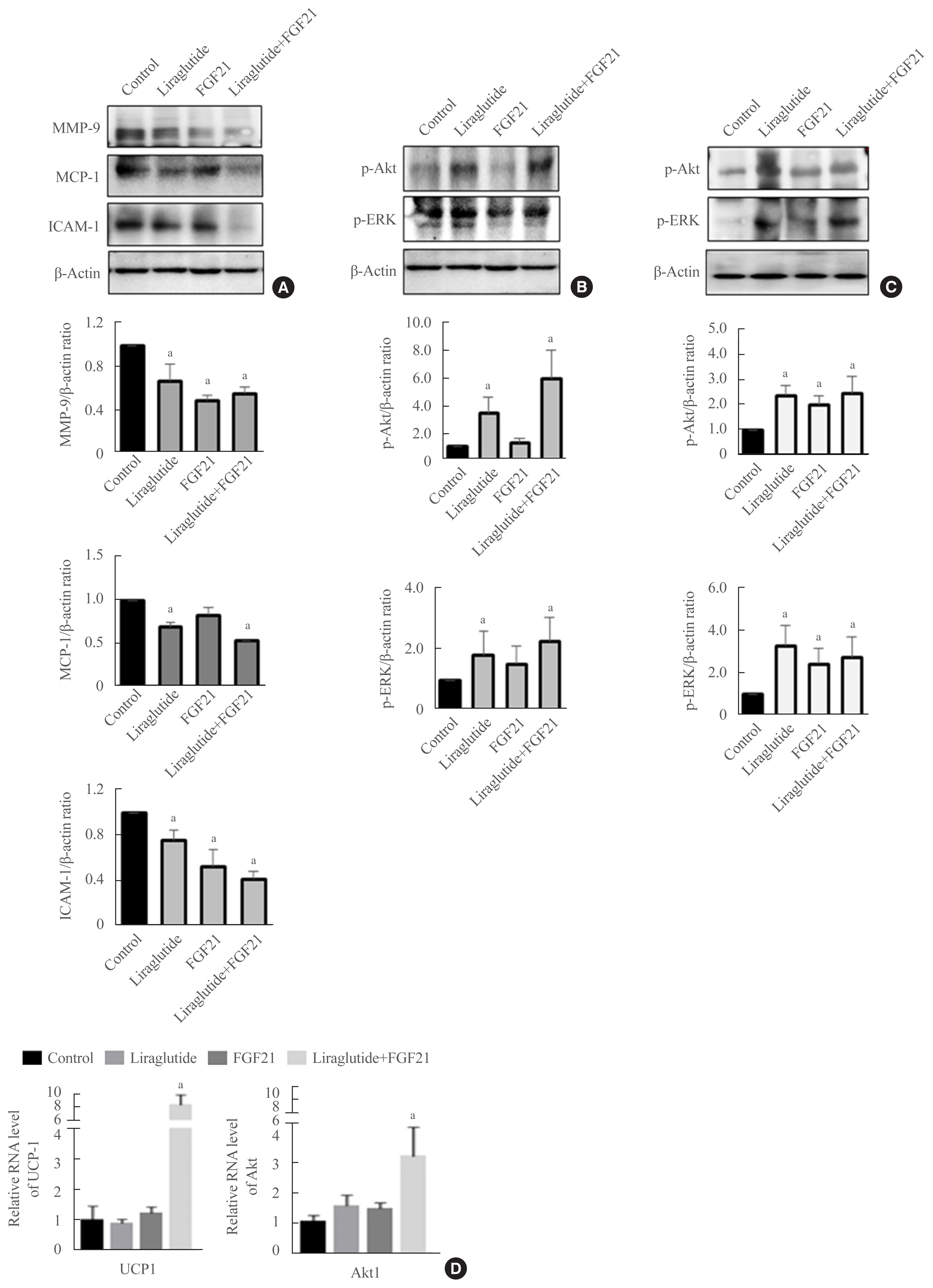
Fig. 5
Liraglutide alone or in combination with fibroblast growth factor 21 (FGF21) inhibits cell proliferation, and migration. (A) A 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay was performed to determine vascular smooth muscle cell (VSMC) viability. VSMCs were exposed to the indicated concentrations of liraglutide or combination treatment for 24 hours, followed by treatment with or without platelet-derived growth factor (PDGF; 10 ng/mL). (B) Quantification of the wound area. The effect of a 24-hour treatment with liraglutide or the liraglutide and FGF21 combination on the migration of VSMCs was determined by an in vitro scratch wound-healing migration assay. Each experiment was performed in triplicate (mean±standard error). aP<0.05.
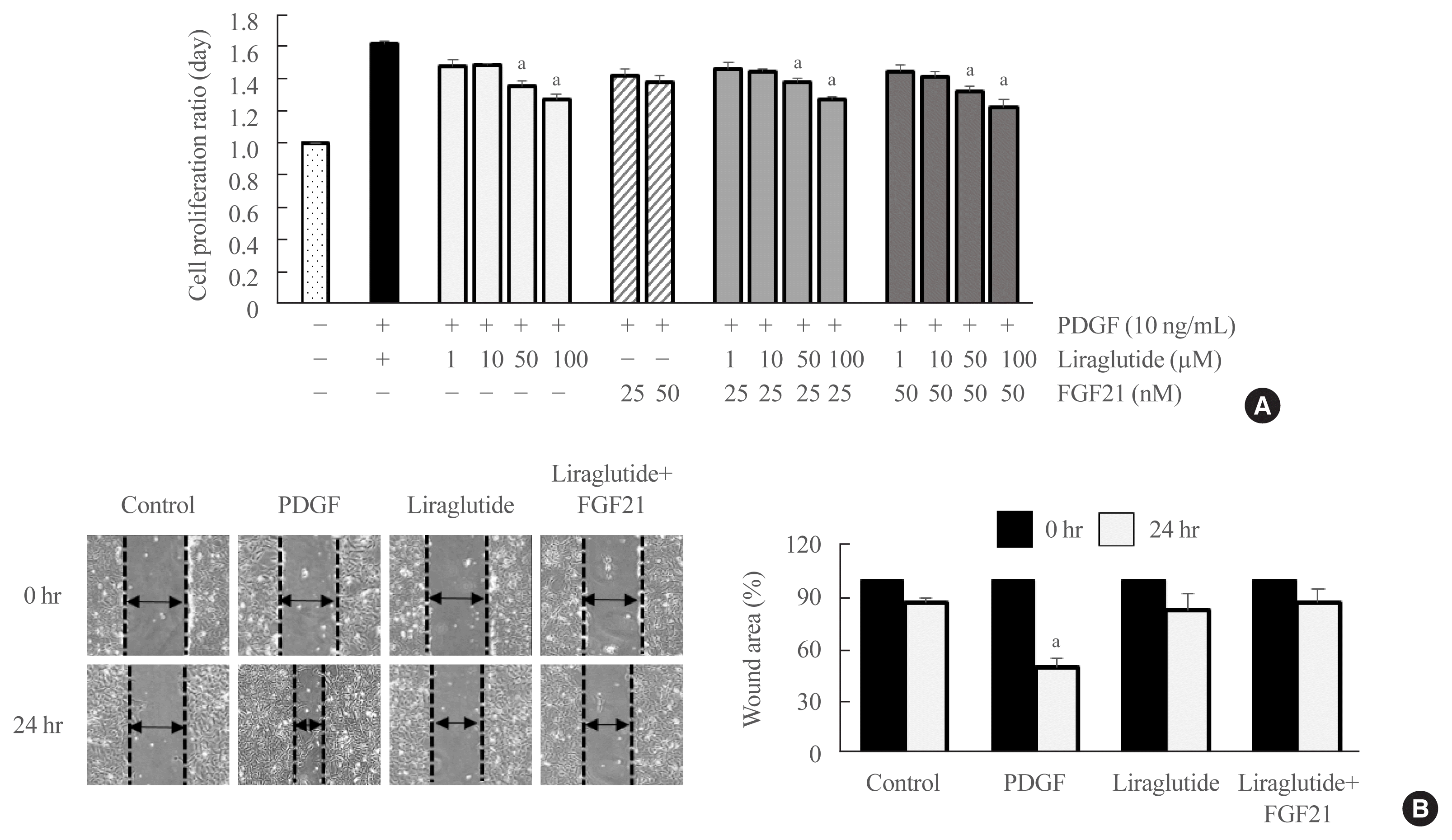
Fig. 6
Effect of liraglutide and fibroblast growth factor 21 (FGF21) on vascular smooth muscle cell (VSMC) apoptosis. (A) Flow cytometry analysis of fluorescein isothiocyanate (FITC)/Annexin V- and propidium iodide-stained VSMCs treated with liraglutide alone (50 μM) or combination of liraglutide (10 μM) and FGF21 (50 nM) for 24 hours. In each scatter plot, the upper-left quadrant shows necrotic cells, the upper-right quadrant shows late apoptotic cells, the lower-left quadrant shows the survival cell mass, and the lower-right quadrant shows early apoptotic cells. (B) Representative three sets of Western blotting bands showing the upregulation of cleaved caspase-3 by liraglutide alone (50 μM) or combination of liraglutide (10 μM) and FGF21 (50 nM) treatment. An anti-cleaved caspase-3 (Asp175) monoclonal antibody was used to detect the large fragment (17/19 kDa) of activated caspase-3 resulting from cleavage adjacent to Asp175. Each experiment was performed in triplicate (mean±standard error). PDGF, platelet-derived growth factor. aP<0.05.
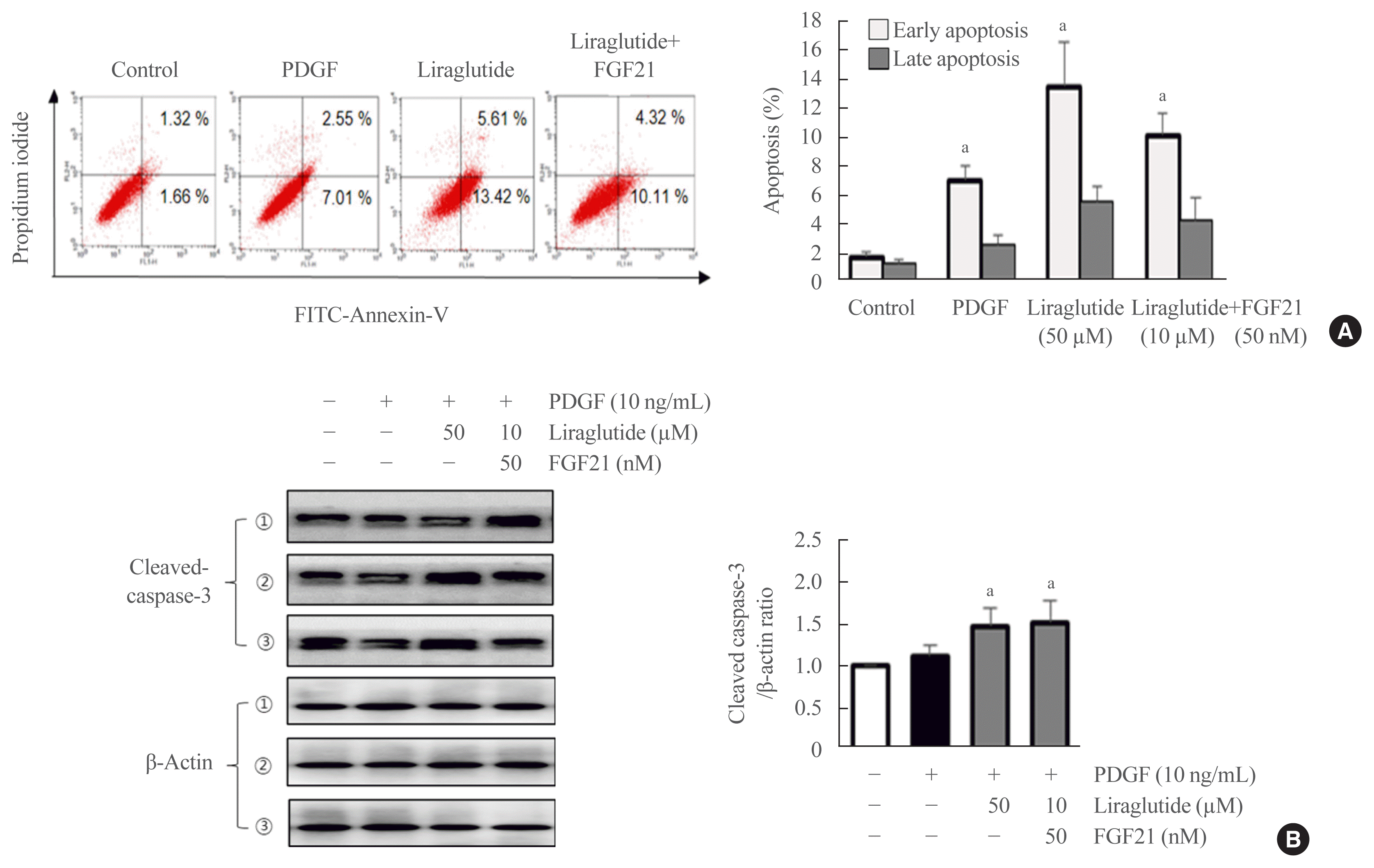
Fig. 7
Effect of liraglutide alone and the liraglutide and fibroblast growth factor 21 (FGF21) combination on inflammation, adhesion molecules, and Akt and extracellular signal-regulated kinase (ERK)1/2 in vascular smooth muscle cells (VSMCs). (A) The decrease in matric mentalloproteinase-9 (MMP-9) and intercellular adhesion molecule-1 (ICAM-1) expression in VSMCs was more pronounced in cells treated with liraglutide and the combination treatment compared with the platelet-derived growth factor (PDGF)-stimulated group. (B) Liraglutide alone and the liraglutide and FGF21 combination treatment significantly suppressed the PDGF-induced phosphorylation of Akt and ERK in VSMCs (mean±standard error). aP<0.05.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download