Abstract
Advances in medical sciences and evidence-based medicine have led to momentous changes in classification and management of thyroid neoplasms. Much progress has been made toward avoiding overdiagnosis and overtreatment of thyroid cancers. The new 2017 World Health Organization (WHO) classification of thyroid neoplasms updated the diagnostic criteria and molecular and genetic characteristics reflecting the biology and behavior of the tumors, and newly introduced the category of borderline malignancy or uncertain malignant potential. Some neoplasms were subclassified, renamed, or redefined as a specific entity. This review introduces changes in the fourth edition WHO classification of thyroid tumors and updates the contemporary diagnosis and classification of thyroid tumors. We also discuss several challenges with the proposal of new diagnostic entities, since they have unique histopathologic and molecular features and clinical relevance.
A histopathological classification of neoplasms reflects the biology and behavior of the tumors and serves as a guide for doctors and clinical management of patients. The World Health Organization (WHO) classification of tumors group publishes the WHO classification of tumors series to provide the international standards for cancer diagnosis including diagnostic criteria, pathological features, and molecular alterations. The WHO classification of tumors of endocrine organs was published in the fourth edition of the WHO series in 2017.
Compared with the third edition of the WHO classification published in 2004 [1], the most significant changes in the 2017 WHO classification of thyroid tumors involve (1) molecular and genetic characterization of follicular-derived thyroid tumors; (2) a new classification for encapsulated well-differentiated follicular tumors, in particular, introduction of tumors of borderline malignancy or uncertain malignant potential; (3) changing the international classification of diseases for oncology (ICD-O) behavior code of hyalinizing trabecular tumor to /1 (borderline or uncertain); (4) identification of new variants of papillary thyroid carcinoma (PTC); (5) subclassification of follicular thyroid carcinoma (FTC) into minimally invasive, encapsulated angioinvasive and widely invasive types; (6) reclassification of Hürthle cell adenoma/carcinoma and poorly differentiated thyroid carcinoma (PDTC) as distinct entities; and (7) changing the term carcinoma showing thymus-like differentiation to intrathyroid thymic carcinoma, as shown in Table 1 [2].
In the ICD-O codes, the morphology code records the histological type of the neoplasm and its biological activity in terms of how it behaves. Behavior codes /0, /1, /2, and /3 mean benign neoplasms, neoplasms of uncertain behavior, carcinoma in situ, and malignant neoplasm, respectively. The behavior code /2 has not been assigned to thyroid tumors. However, not all histologic types of the neoplasms have specific ICD-O code numbers. In the 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10), the topography codes classify neoplasms according to their original site, assign malignant neoplasms to category C, and assign other neoplasms (in situ neoplasms, benign neoplasms, and neoplasms of uncertain or unknown behavior) to category D. Table 2 shows the ICD-10 and ICD-O codes of thyroid tumors according to the 2017 WHO classification.
In this review we briefly introduces a historical perspective on histopathological classification of thyroid tumors, changes in the fourth edition of the WHO classification of thyroid tumors, and update the contemporary diagnosis and classification of thyroid tumors since the revision of the WHO classification in 2017.
There were revisions on diagnostic criteria for PTC in almost every edition of the WHO thyroid tumor classification. In the first edition (1974), PTC was a malignant epithelial tumor containing papillary structure (regardless of nuclear features). PTC-type nuclear features became essential criteria for malignancy in the second (1988) and third (2004) editions.
In the second edition, PTC was a malignant epithelial tumor showing evidence of follicular cell differentiation, typically with papillary and follicular structures as well as characteristic nuclear changes.
In the third edition, PTC was a malignant epithelial tumor showing evidence of follicular cell differentiation and a set of distinctive nuclear features. However, precise morphological criteria of distinctive nuclear features for PTCs were not provided to encourage a good consensus among pathologists, which resulted in severe interobserver variation in the diagnosis of PTCs.
In the fourth edition, a borderline tumor entity was incorporated to reduce the diagnostic discordance, and a nuclear score guide for RAS type PTC was provided. It was also aimed to reduce overdiagnosis and overtreatment of low-risk PTCs. The fourth edition further modified the definition of PTC and stated that “PTC is a malignant epithelial tumor showing evidence of follicular cell differentiation and a set of distinctive nuclear features. PTC is usually invasive. Papillae, invasion, or cytological features of PTC are required.” These changes were also intended to reduce overdiagnosis and overtreatment of low-risk PTCs, and the majority of encapsulated PTCs without clear-cut invasion were downgraded to the borderline tumor category, including well-differentiated tumor of uncertain malignant potential (WDT-UMP) and noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP), because PTCs were still heterogeneous tumors genetically, and the nuclear feature alone was not successfully correlated with genetic alterations (RET/PTC rearrangements, BRAF V600E mutation, and RAS mutations) and accurate identification of the true biological nature of the tumors.
In the fourth edition WHO classification, FTC was classified into three prognostic groups: (1) minimally invasive (capsular invasive only) FTC; (2) encapsulated angioinvasive FTC; and (3) widely invasive FTC. Furthermore, in the follicular adenoma (FA)/FTC lineage, significant numbers of low-risk FTC (minimally invasive FTC) were downgraded to the borderline tumor category (follicular tumor of uncertain malignant potential [FT-UMP]) using stricter criteria of capsular and vascular invasions. It was also intended to reduce overtreatment for low-risk FTCs.
There were several breakthrough events in thyroid tumor classification recently. The introduction of borderline tumors (hyalinizing trabecular tumor, NIFTP, and tumors of uncertain malignant potential) to thyroid tumor classification is an epoch-making change in thyroid pathology practice [3–8]. With this breakthrough change, thyroid tumors are classified into three risk groups by the probability of recurrence/metastasis: negligible risk (<0.1%) in benign tumors, very low risk (<1%) in borderline tumors, and high risk in malignant tumors [2]. As a consequence, the definition of FA was altered in the new 2017 WHO classification of thyroid tumors [2]. It states that FA is a benign, encapsulated, noninvasive neoplasm showing evidence of thyroid follicular cell differentiation, without nuclear features of PTC [2]. A new phrase, “without nuclear features of PTC,” was added to the previous definition by the 2004 WHO classification of thyroid tumors, because noninvasive encapsulated follicular pattern tumors with PTC-like nuclear features are excluded from FA and are classified in NIFTP or WDT-UMP in the new definition (Fig. 1) [2].
There were several excellent articles and reviews that analyzed the effects of NIFTP on thyroid practice [2,7,9–22]. However, they focused on diagnosis, prevalence, and prognosis of NIFTP and did not emphasize the significant modification in the diagnosis of FA. Furthermore, several studies reported significant observer variation in PTC-type nuclear features and PTC-like nuclear features in the diagnosis of encapsulated follicular pattern thyroid tumors [21,23–25], which also should affect FA diagnosis.
In this review article, we would like to point out different handlings of the two types of nuclear features between Asian and Western thyroid practice [26]. They were called either (1) PTC-type nuclear features that have fully developed PTC-type nuclear changes (often observed in BRAF V600E mutated PTCs) and (2) PTC-like nuclear features (delicate nuclear changes) defined by Nikiforov et al. [8] using the nuclear score guide that were often seen in RAS mutated PTCs and NIFTP (Fig. 1). All of them were encapsulated follicular patterned thyroid tumors, and most review articles did not mention how to handle encapsulated papillary patterned tumors in detail, because tumors with true papillae were excluded from the NIFTP diagnosis in the definition [2,7,8,18]. However, differentiating between FA and noninvasive encapsulated PTC is a challenge for pathologists, because the differential diagnosis includes FA with papillary hyperplasia and noninvasive encapsulated classic PTC in addition to NIFTP, FT-UMP, WDT-UMP, and encapsulated follicular variant of PTC (Fig. 2).
Observer variations in diagnosing those thyroid tumors have been reported to be significant [21,23–26]. There should be borderline/precursor thyroid tumors with a papillary growth pattern that was not clearly defined in the current WHO classification (Fig. 3) [27]. Ohba et al. [27] recently proposed a new borderline thyroid tumor with a papillary growth pattern and named it noninvasive encapsulated papillary RAS-like thyroid tumor (NEPRAS) (Fig. 4). Subsequent studies reported more cases of NEPRAS [28,29].
How to classify noninvasive encapsulated thyroid tumors into encapsulated PTC, NIFTP, NEPRAS, and FA, as we have recommended, is shown in Fig. 5 using nuclear features (BRAF-type PTC nuclear features or RAS-type papillary-like nuclear features) and growth patterns (follicular growth or papillary growth).
A hyalinizing trabecular tumor is a rare thyroid neoplasm of follicular cell origin with very low malignant potential. The tumor was moved from a benign group of “thyroid adenoma and related tumors” in the 2004 WHO classification to a separate category, where it is classified as a neoplasm of uncertain behavior with an ICD-O behavior code of /1 in the 2017 WHO classification [2]. Hyalinizing trabecular tumors have a very good prognosis. To date, only one case with lung metastasis has been reported [4], and regional lymph node metastases are reported in another case [30]. However, these cases with adverse outcomes have been criticized as a misdiagnosis of encapsulated solid/trabecular PTC.
Because hyalinizing trabecular tumors have PTC-type nuclear features, including intranuclear grooves and pseudoinclusions, they can be challenging to diagnose in fine-needle aspiration cytology (Fig. 6). More than half of the tumors were diagnosed as PTC or suspicious for PTC [31].
Although RET/PTC fusions characteristic of PTC were reported in early studies [4], subsequent studies confirmed no RET/PTC rearrangements in the tumors using more accurate molecular diagnostic methods [31]. Recent studies reported the PAX8/GLIS fusions to be a genetic hallmark of hyalinizing trabecular tumor [31,32]. No PAX8/GLIS fusions have been found in other types of thyroid tumors to date [31–34]. Because all hyalinizing trabecular tumors with fully developed histopathologic features of the tumor had PAX8/GLIS fusions and benign clinical behavior, Nikiforova et al. [34] suggested that the diagnostic term for these tumors should be “GLIS-rearranged hyalinizing trabecular adenoma” changing the current term of “tumor” to “adenoma.”
PTC had been the exclusive malignancy that could be diagnosed merely by its characteristics of cell nuclear features regardless of the tumor structures and growth patterns, until NIFTP was nominated and separated from the category of PTC, encapsulated follicular variant [2]. The addition of “PTC is usually invasive” in the definition of PTC suppresses the diagnosis of encapsulated tumors to PTC. This change may be in parallel with the construction of a new borderline tumor category. The diagnostic criteria for PTC allow it to demonstrate various histological features and growth patterns; so many PTC variants are recognized, including classic, microcarcinoma (≤1 cm in diameter), encapsulated, follicular, diffuse sclerosing, tall cell, columnar cell, cribriform-morular, hobnail, PTC with fibromatosis/fasciitis-like stroma, solid/trabecular, oncocytic, spindle cell, clear cell, and Warthin-like variants (Table 3) [2].
Among PTC variants, tall cell, columnar cells, and hobnail variants are of undoubted clinical significance, since they are aggressive variants associated with aggressive clinicopathological features and worse prognosis than for classic and encapsulated PTC [2,35,36]. In the 2015 American Thyroid Association risk stratification system, patients with these three aggressive variants are classified as having an intermediate risk of recurrence [37].
Although solid and diffuse sclerosing variants are regarded as aggressive variants of PTC by some researchers, their biological behaviors are still controversial [2,35,36]. An increase in the incidence of aggressive PTCs was observed at a rate higher than that seen in well-differentiated PTCs or anaplastic thyroid carcinomas (ATCs) in the past two decades in a study of a large cohort of thyroid cancers [36]. There is increasing evidence that when aggressive variants of PTCs present as low stage and without invasive features (including extrathyroid invasion, tumor thrombi, and lymph node metastasis), they might seem rather indolent [38,39].
The definition of the tall cell variant was revised in the current WHO classification, because at least 30% of tumor cells are two to three times as tall as they are wide (Fig. 7), whereas in the former edition of the WHO classification, the tall cell variant needed to have predominant tumor cells three times as tall as they are wide [1,2,40]. The revision in the definition has allowed more cases of PTC to be included in this variant, and could predict disease-free survival more precisely [41,42].
There is increasing evidence that PTCs with as little as 10% tall cell change have a worse prognosis than do those without tall cells, and that PTCs with focal tall cell change (≥10%) should be reported and handled beyond the low-risk classification [41,43–47]. The diagnosis of the tall cell variant should not be missed, in that patients with this variant are more refractory to radioactive iodine ablation and have a worse prognosis than do those with classic PTC [36,48–50]. BRAF V600E and TERT promotor mutations are the most common genetic alterations for this variant [44,51].
The columnar cell variant consists of columnar cells with marked pseudostratified hyperchromatic nuclei and absence of conventional PTC nuclear features (Fig. 8) [2,40]. It is an interesting finding that CDX2, a gut-specific nuclear transcription factor, the expression of which is a putative feature of intestinal-type differentiation, is positive in 10% to 55% of this variant [52,53], which may behave indolently or aggressively depending on tumor encapsulation and tumor size [54,55]. Columnar cell variants of circumscribed or intrathyroidal types have an excellent prognosis, whereas invasive tumors with extrathyroidal extension have a less favorable prognosis [50,56].
The hobnail variant is a novel entity in the 2017 WHO classification of tumors of endocrine organs, defined by >30% of tumor cells with hobnail features [2]. It is rare and accounts for <3% of all PTC cases [57]. Hobnail features in PTC were firstly described by Kakudo et al. [58] in 2004 as loss of cellular polarity and cohesiveness, with the main growth pattern of micropapillary and papillary structure, which was correlated with a higher risk of recurrence. In the following years, several articles concerning loss of cellular polarity and cohesiveness, micropapillary, or hobnail features in PTC were published, further clarifying that this feature represented an aggressive growth and was correlated with poor prognosis, in that most cases had lymph node/distant metastases and local recurrence, although different cut-off values of hobnail features were used in different studies, from two unequivocal points in the invasive front to 30% of total tumor cells [59–63]. An epithelial-mesenchymal transition was suggested as a possible mechanism of metastasis for this variant [60,62,64]. BRAF V600E mutation is the most common genetic alteration for this variant, accounting for 50% to 94% cases, followed by TP53 mutation [57,61,65–67].
The hobnail variant must be differentiated from classic PTCs showing hobnail-like morphology, which is associated with papillae with hyalinized and edematous fibrovascular core (Fig. 9) [68]. The hobnail-like morphology in classic PTC with cystic change is not a true hobnail variant but degenerative changes.
The solid variant should be identified when most of the tumor consists of solid/trabecular/nested components and other variants are ruled out [2]. A subset of solid variant PTCs, which lack conventional PTC nuclear features and present high mitotic figures and/or necrosis, fulfill the diagnostic criteria for PDTC and therefore are currently classified as PDTC [2,69].
This variant is strongly associated with ionizing radiation and is more common in young patients and children [70–73]. Radiation-associated pediatric cases frequently show RET/PTC3 rearrangement and sporadic cases show RET/PTC1/3 and ETV6/NTRK3 fusions [72,74]. BRAF V600E mutations are less commonly found than are classic PTC.
The diffuse sclerosing variant, which usually presents with rapid and diffuse enlargement of the thyroid gland, clinically may be mistaken for autoimmune thyroiditis. It is most commonly seen in young women and is characterized by marked stromal fibrosis, dense lymphocytic infiltration, numerous psammoma bodies, and foci of squamous metaplasia, as well as frequent lymphovascular invasion [2,76]. RET/PTC rearrangements represented the genetic initiation event for this variant [77–79]. The BRAF V600E mutation is rare in this variant.
This variant is associated with extrathyroidal extension, cervical lymph node and distant metastasis, and shorter disease-free survival; however, the mortality rate is comparable to that of the classic variant [80–83]. The excellent long-term survival despite a higher recurrence rate may result from the favorable effect of a younger patient age.
FTC is the second common malignancy with follicular cell differentiation, which lacks the diagnostic nuclear features of PTC. In the 2004 WHO classification, FTC was divided into minimally invasive and widely invasive, according to the capsular invasion extent. In the 2017 WHO classification, minimally invasive FTC is further classified as minimally invasive (capsular invasion only) and encapsulated angioinvasive (with or without capsular invasion) (Fig. 10). There is no revision in the diagnostic criteria for widely invasive FTC, which extensively invades the thyroid gland and extra-thyroidal soft tissue and often shows extensive vascular invasion. However, extensive angioinvasion alone does not categorize the FTC as being widely invasive FTC.
The extent of vascular invasion in FTC is prognostically relevant [2,84–86]. In encapsulated angioinvasive FTC, tumors with limited vascular invasion (<4 vessels) have a better outcome than do those with extensive vascular invasion (≥4 vessels) [84,85]. However, extensive vascular invasion did not affect the survival of patients with widely invasive FTC independently [84]. The TERT promoter mutation was seen in FTC, but not in atypical FA or FT-UMP, and the TERT promoter mutation was significantly higher in patients with metastatic FTC than in patients with non-metastatic FTC [87].
Hürthle cell tumors, including Hürthle cell adenomas and Hürthle cell carcinomas, should be diagnosed if the majority of the tumor (>75%) consists of oncocytic cells [2,44]. Hürthle cell adenomas and carcinomas had been regarded as variants of FA and FTC, respectively, but they were nominated as a distinct entity in the 2017 WHO classification, since they have biological behaviors and molecular profiles different from those of their counterparts among follicular tumors (Fig. 11) [85,88–92]. Hürthle cell carcinomas are different from conventional FTCs, in that Hürthle cell carcinomas occur in older patients, are more common in men, can develop cervical lymph node metastasis, and have larger tumors, higher-stage disease, and worse prognosis, and are resistant to radioactive-iodine treatment [93]. As with conventional FTC, the prognosis of Hürthle cell carcinoma is related to the extent of vascular invasion [85].
Integrated genomic analysis of 56 Hürthle cell carcinomas revealed that, besides recurrent mitochondrial mutations, whole-chromosomal duplications of chromosomes 5 and 7 and widespread loss of heterozygosity because of haploidization and copy-number-neutral uniparental disomy were unique chromosomal landscapes for this special tumor [94]. EZH1 mutation was identified in a small subset of Hürthle cell adenoma and minimally invasive Hürthle cell carcinoma, but not in widely invasive Hürthle cell carcinoma [95].
PDTC is a follicular cell neoplasm that is morphologically and behaviorally intermediate between well-differentiated thyroid carcinoma (WDTC) and ATC. Different definitions of PDTC have been reported. The most well-known ones are Sakamoto-type PDTC, Memorial Sloan Kettering Cancer Center (MSKCC) criteria, and the Turin proposal [69,96,97]. Sakamoto et al. [96] focused on the tumor growth pattern (solid, trabecular, and/or scirrhous) in 1983. In the 2004 WHO classification, PDTC was vaguely defined as a “follicular-cell neoplasms that show limited evidence of structural follicular cell differentiation and both morphologically and behaviorally occupy an intermediate position between differentiated (follicular and papillary carcinomas) and undifferentiated (anaplastic) carcinomas” [1]. The MSKCC criteria defined PDTC based on the tumor necrosis and/or mitotic figures (≥5/10 high-power fields) in 2006 [97]. In 2007, the Turin proposal covered the definitions by Sakamoto et al. and MSKCC criteria, and made a revision [69,96,97].
The 2017 WHO classification adopted the Turin consensus proposal as the diagnostic criteria for PDTC, including (1) diagnosis of carcinoma of follicular cell origin; (2) a solid, trabecular, or insular growth pattern; (3) absence of conventional nuclear features of PTC; and (4) at least one of the following three features: convoluted nuclei, ≥3 mitoses per 10 high-power fields, or tumor necrosis [2,69]. The convoluted nuclei refer to raisin-like hyperchromatic nuclei with irregular membranes and may reflect dedifferentiation of PTC [69], but they are also considered by some pathologists to be degenerated nuclei without nuclear features of PTC.
Validation studies confirmed that the Turin consensus criteria could reliably select PDTC cases, and they reflected the patient prognosis more accurately than that in the 2004 WHO classification [13,98]. Although this algorithm was not initially used for Hürthle (oncocytic) cell carcinoma, it was proved to be practical and is now accepted as being useful for identifying oncocytic PDTC [2,99].
The Ki-67 index is a helpful marker used adjunctively in differentiating PDTC from WDTC and ATC, since it is usually 10% to 30% in PDTC, but <10% in WDTC and >30% in ATC [2,100–102]. Kakudo et al. [101] also proposed a prognostic classification of thyroid tumors into benign, borderline, and malignant tumors by the Ki-67 labelling index (Table 4). They proposed “high-risk thyroid carcinoma of follicular cell origin” defined by the Ki-67 index of 10% to 30% to cover all histological types of high-risk carcinomas, listed in the WHO classification as one of the synonyms and related terms of PDTC.
PDTC could be pure or could coexist with PTC or other types of tumors. In the latter case, the percentage of PDTC should be reported. As little as 10% of PDTC in a background of well-differentiated tumors could be associated with aggressive features and unfavorable prognosis [103]. The extent of invasion was an important parameter that affected clinical outcomes for patients with PDTC, and patients with encapsulated PDTC with capsular invasion only or focal vascular invasion (fewer than four foci) had an excellent outcome [104].
In accordance with its morphology and biological behavior, PDTC has genetic alterations embracing the alterations in PTC, FTC, and ATC: BRAF V600E, RAS, TERT promoter, TP53 mutations, and other high-risk gene mutations. The TERT promotor mutation represents the most common genetic alteration [105]. The prevalence of the TERT promoter mutation was significantly higher in tumors harboring aggressive histological features (including PDTC, tall cell variant of PTC, and widely invasive FTC) than in non-aggressive thyroid carcinomas, and TERT promoter mutations were associated with poor outcomes [106].
Mitotic figures and necrosis are uncommon in PTC and FTC. Typically, mitoses are found to be less than three per 10 high-power fields in the WDTC. The presence of high mitotic rates and/or necrosis raises the possibility of PDTC. However, according to the WHO classification, the high-grade features alone do not categorize the tumor as PDTC (Fig. 12). The same tumors can be classified as PDTC by the MSKCC criteria if tumor necrosis and/or mitoses of ≥5 per 10 high-power fields are found in a follicular-derived carcinoma regardless of growth pattern [97,107].
ATC is a highly aggressive malignancy that usually presents as advanced disease for which even radical surgery could not be possible. ATC can develop through dedifferentiation of a differentiated thyroid carcinoma or directly from follicular cells. Favorable patient survival was reported in cases with small foci of ATCs found incidentally within WDTCs after thyroid surgery [108]. Therefore, the percentage of ATC components should be noted in the pathology report.
Histologically, ATC can consist of one or in any combination of three patterns: sarcomatoid (composed of spindle cells), giant cell (composed of multinucleated giant cells), and epithelial (composed of squamoid or squamous tumor cells). All forms of ATC are characterized by a dense inflammatory infiltrate, tumor-infiltrating macrophages, necrosis, and a high mitotic rate. The rare histologic variants of ATC include paucicellular, rhabdoid, and small cell variants.
The diagnosis of ATC is challenging, since it overlaps morphologically with other malignancies showing anaplastic features, and the diagnoses should be made only from biopsy materials in most circumstances [102,109,110]. The rational use of thyroid-specific markers as well as other tissue-specific markers is helpful in differentiating ATC from its mimics [102,109,110]. PAX8 is reported to be a sensitive marker for detecting cells of thyroid origin other than TTF1 and thyroglobulin [109,111]. Recently, a multi-institutional study demonstrated that PAX8, detected by a monoclonal antibody MRQ-50 used worldwide, was positively expressed in 54.4% of 182 ATCs [112]. CD10 is diffusely and strongly expressed in ATC but absent or focally in WDTCs (Fig. 13) [113,114].
In a recent large retrospective cohort study of 360 cases of ATC, the TERT promoter mutation was most prevalent (75%), followed by TP53 (63%), BRAF (45%), RAS (24%), PIK3CA (18%), E1F1AX (14%), and PTEN mutations (14%) [115]. Concomitant TERT promoter and BRAF/RAS mutations were associated with worse outcomes than were mutation in only one of the genes [115].
Thymic and third pharyngeal pouch remnants entrapped in the thyroid gland can give rise to ectopic thymoma, spindle epithelial tumor with thymus-like differentiation, and intrathyroid thymic carcinoma. The current 2017 WHO classification changed the term “carcinoma showing thymus-like differentiation” in the 2004 WHO classification to “intrathyroid thymic carcinoma” [1,2].
Intrathyroid thymic carcinomas share the immunohistochemical profile of primary thymoma that is positive for cytokeratin, CD5, KIT (also known as c-Kit and CD117), and p63, and negative for thyroglobulin and TTF1. Three histologic subtypes of this tumor include the squamous cell carcinoma type, lymphoepithelioma or basaloid type, and neuroendocrine carcinoma type.
NTRK gene fusions found in the thyroid tumors involve NTRK1 and NTRK3 genes encoding the neurotrophin receptors TrkA and TrkC, respectively [33,116–118]. The most common NTRK fusion identified in PTC is ETV6-NTRK3 and less frequently identified fusions are TPM3-NTRK1, TPR-NTRK1, and TFG-NTRK1 [119]. The detection of ETV6-NTRK3 fusion is useful for the diagnosis of secretory carcinomas occurring in the breast, salivary gland, and thyroid gland. To date, all cases of secretory carcinomas of the thyroid gland have an ETV6-NTRK3 fusion only. Secretory carcinomas show various histologic patterns, including microcystic, cribriform, solid, papillary, and tubular growth. Colloid-like secretions are found in the intraluminal and cystic spaces. Tumor cells are positive for S100, mammaglobulin, GATA-3, and PAX8, but negative for thyroglobulin and TTF-1, helping to differentiate the tumor from PTC. Although this new entity has not been listed in the WHO classification of tumors of endocrine organs, cancer-type classification based on genetic alterations is clinically relevant, given that tyrosine kinase inhibitors targeting somatic mutations and fusions have been approved for the treatment of cancer patients [120].
Binary classification of benign or malignant thyroid neoplasms has raised many clinical problems of overdiagnosis and unnecessary treatment of thyroid cancers. The 2017 WHO classification categorized thyroid neoplasms into benign, borderline (uncertain malignant potential), and malignant tumors. Certain types of thyroid cancers were reclassified as borderline tumors. Histopathologic variants of PTC and FTC were redefined to better stratify prognosis and management of patients. Molecular-based classification for thyroid neoplasm allows diagnosis and prognostic risk stratification of thyroid cancer and may increase the accessibility of targeted treatments. As always, the current WHO classification of thyroid neoplasms will not be perfect, but it represents the current best-practice statements. Attempts to create a new classification system for thyroid tumors have also been made to improve the current risk stratification system for thyroid cancers.
ACKNOWLEDGMENTS
This research was supported by a grant (NRF-2020R1F1-A1070028) from the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science and ICT. The authors gratefully acknowledge iKooB Inc. for help with figures.
REFERENCES
1. Delellis RA, Lloyd RV, Heitz PU, Eng C. World Health Organization classification of tumours of endocrine organs. 3rd ed. Lyon: International Agency for Research on Cancer (IARC);2004. p. 49–123.
2. Lloyd RV, Osamura RY, Kloppel G, Rosai J. WHO classification of tumours of endocrine organs. 4th ed. Lyon: International Agency for Research on Cancer (IARC);2017. p. 65–143.
3. Williams ED. Guest editorial: two proposals regarding the terminology of thyroid tumors. Int J Surg Pathol. 2000; 8:181–3.

4. Carney JA, Hirokawa M, Lloyd RV, Papotti M, Sebo TJ. Hyalinizing trabecular tumors of the thyroid gland are almost all benign. Am J Surg Pathol. 2008; 32:1877–89.

5. Kakudo K, Bai Y, Liu Z, Li Y, Ito Y, Ozaki T. Classification of thyroid follicular cell tumors: with special reference to borderline lesions. Endocr J. 2012; 59:1–12.

6. Kakudo K, Bai Y, Liu Z, Ozaki T. Encapsulated papillary thyroid carcinoma, follicular variant: a misnomer. Pathol Int. 2012; 62:155–60.

7. Kakudo K, El-Naggar AK, Hodak SP, Khanafshar E, Nikiforov YE, Nose V, et al. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) in thyroid tumor classification. Pathol Int. 2018; 68:327–33.

8. Nikiforov YE, Seethala RR, Tallini G, Baloch ZW, Basolo F, Thompson LD, et al. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: a paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol. 2016; 2:1023–9.

9. Baloch ZW, Seethala RR, Faquin WC, Papotti MG, Basolo F, Fadda G, et al. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP): a changing paradigm in thyroid surgical pathology and implications for thyroid cytopathology. Cancer Cytopathol. 2016; 124:616–20.

10. Bychkov A, Hirokawa M, Jung CK, Liu Z, Zhu Y, Hong SW, et al. Low rate of noninvasive follicular thyroid neoplasm with papillary-like nuclear features in Asian practice. Thyroid. 2017; 27:983–4.

11. Bychkov A, Keelawat S, Agarwal S, Jain D, Jung CK, Hong S, et al. Impact of non-invasive follicular thyroid neoplasm with papillary-like nuclear features on the Bethesda system for reporting thyroid cytopathology: a multi-institutional study in five Asian countries. Pathology. 2018; 50:411–7.

12. Cho U, Mete O, Kim MH, Bae JS, Jung CK. Molecular correlates and rate of lymph node metastasis of non-invasive follicular thyroid neoplasm with papillary-like nuclear features and invasive follicular variant papillary thyroid carcinoma: the impact of rigid criteria to distinguish non-invasive follicular thyroid neoplasm with papillary-like nuclear features. Mod Pathol. 2017; 30:810–25.

13. Akaishi J, Kondo T, Sugino K, Ogimi Y, Masaki C, Hames KY, et al. Prognostic impact of the turin criteria in poorly differentiated thyroid carcinoma. World J Surg. 2019; 43:2235–44.

14. Ibrahim AA, Wu HH. Fine-needle aspiration cytology of noninvasive follicular variant of papillary thyroid carcinoma is cytomorphologically distinct from the invasive counterpart. Am J Clin Pathol. 2016; 146:373–7.

15. Maletta F, Massa F, Torregrossa L, Duregon E, Casadei GP, Basolo F, et al. Cytological features of “noninvasive follicular thyroid neoplasm with papillary-like nuclear features” and their correlation with tumor histology. Hum Pathol. 2016; 54:134–42.

16. Parente DN, Kluijfhout WP, Bongers PJ, Verzijl R, Devon KM, Rotstein LE, et al. Clinical safety of renaming encapsulated follicular variant of papillary thyroid carcinoma: is NIFTP truly benign? World J Surg. 2018; 42:321–6.

17. Rosario PW, Mourao GF. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP): a review for clinicians. Endocr Relat Cancer. 2019; 26:R259–66.

18. Seethala RR, Baloch ZW, Barletta JA, Khanafshar E, Mete O, Sadow PM, et al. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features: a review for pathologists. Mod Pathol. 2018; 31:39–55.

19. Strickland KC, Vivero M, Jo VY, Lowe AC, Hollowell M, Qian X, et al. Preoperative cytologic diagnosis of noninvasive follicular thyroid neoplasm with papillary-like nuclear features: a prospective analysis. Thyroid. 2016; 26:1466–71.

20. Thompson LD. Ninety-four cases of encapsulated follicular variant of papillary thyroid carcinoma: a name change to noninvasive follicular thyroid neoplasm with papillary-like nuclear features would help prevent overtreatment. Mod Pathol. 2016; 29:698–707.

21. Thompson LD, Poller DN, Kakudo K, Burchette R, Nikiforov YE, Seethala RR. An international interobserver variability reporting of the nuclear scoring criteria to diagnose noninvasive follicular thyroid neoplasm with papillary-like nuclear features: a validation study. Endocr Pathol. 2018; 29:242–9.

22. Yang GC, Fried KO, Scognamiglio T. Sonographic and cytologic differences of NIFTP from infiltrative or invasive encapsulated follicular variant of papillary thyroid carcinoma: a review of 179 cases. Diagn Cytopathol. 2017; 45:533–41.
23. Hirokawa M, Carney JA, Goellner JR, DeLellis RA, Heffess CS, Katoh R, et al. Observer variation of encapsulated follicular lesions of the thyroid gland. Am J Surg Pathol. 2002; 26:1508–14.

24. Liu Z, Bychkov A, Jung CK, Hirokawa M, Sui S, Hong S, et al. Interobserver and intraobserver variation in the morphological evaluation of noninvasive follicular thyroid neoplasm with papillary-like nuclear features in Asian practice. Pathol Int. 2019; 69:202–10.

25. Lloyd RV, Erickson LA, Casey MB, Lam KY, Lohse CM, Asa SL, et al. Observer variation in the diagnosis of follicular variant of papillary thyroid carcinoma. Am J Surg Pathol. 2004; 28:1336–40.

26. Kakudo K, Liu Z, Bychkov A, Jung CK. Thyroid FNA cytology, differential diagnoses and pitfalls. 2nd ed. Singapore: Springer;2019. Chapter 21, Nuclear features of papillary thyroid carcinoma (BRAF-like tumors), noninvasive follicular thyroid neoplasm with papillary-like nuclear features (RAS-like tumors) and follicular adenoma/follicular thyroid carcinoma (RAS-like tumors). p. 173–9.
27. Ohba K, Mitsutake N, Matsuse M, Rogounovitch T, Nishino N, Oki Y, et al. Encapsulated papillary thyroid tumor with delicate nuclear changes and a KRAS mutation as a possible novel subtype of borderline tumor. J Pathol Transl Med. 2019; 53:136–41.

28. Jung CK, Park SY, Kim JH, Kakudo K. New insights into classification and risk stratification of encapsulated thyroid tumors with a predominantly papillary architecture. J Pathol Transl Med. 2020; 54:197–203.

29. Rosario PW. Noninvasive encapsulated papillary RAS-like thyroid tumor (NEPRAS) or encapsulated papillary thyroid carcinoma (PTC). J Pathol Transl Med. 2020; 54:263–4.

30. Sambade C, Franssila K, Cameselle-Teijeiro J, Nesland J, Sobrinho-Simoes M. Hyalinizing trabecular adenoma: a misnomer for a peculiar tumor of the thyroid gland. Endocr Pathol. 1991; 2:83–91.

31. Nikiforova MN, Nikiforov YE, Ohori NP. GLIS rearrangements in thyroid nodules: a key to preoperative diagnosis of hyalinizing trabecular tumor. Cancer Cytopathol. 2019; 127:560–6.
32. Marchio C, Da Cruz Paula A, Gularte-Merida R, Basili T, Brandes A, da Silva EM, et al. PAX8-GLIS3 gene fusion is a pathognomonic genetic alteration of hyalinizing trabecular tumors of the thyroid. Mod Pathol. 2019; 32:1734–43.

33. Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014; 159:676–90.
34. Nikiforova MN, Nikitski AV, Panebianco F, Kaya C, Yip L, Williams M, et al. GLIS rearrangement is a genomic hallmark of hyalinizing trabecular tumor of the thyroid gland. Thyroid. 2019; 29:161–73.

35. Nath MC, Erickson LA. Aggressive variants of papillary thyroid carcinoma: hobnail, tall cell, columnar, and solid. Adv Anat Pathol. 2018; 25:172–9.

36. Ho AS, Luu M, Barrios L, Chen I, Melany M, Ali N, et al. Incidence and mortality risk spectrum across aggressive variants of papillary thyroid carcinoma. JAMA Oncol. 2020; 6:706–13.

37. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016; 26:1–133.

38. Song E, Jeon MJ, Oh HS, Han M, Lee YM, Kim TY, et al. Do aggressive variants of papillary thyroid carcinoma have worse clinical outcome than classic papillary thyroid carcinoma? Eur J Endocrinol. 2018; 179:135–42.

39. Limberg J, Ullmann TM, Stefanova D, Buicko JL, Finnerty BM, Zarnegar R, et al. Does aggressive variant histology without invasive features predict overall survival in papillary thyroid cancer?: a national cancer database analysis. Ann Surg. 2019. Oct. 9. [Epub]. https://doi.org/10.1097/SLA.00000-00000003632
.

40. Jung CK. Papillary thyroid carcinoma variants with tall columnar cells. J Pathol Transl Med. 2020; 54:123.

41. Ganly I, Ibrahimpasic T, Rivera M, Nixon I, Palmer F, Patel SG, et al. Prognostic implications of papillary thyroid carcinoma with tall-cell features. Thyroid. 2014; 24:662–70.

42. Wong KS, Higgins SE, Marqusee E, Nehs MA, Angell T, Barletta JA. Tall cell variant of papillary thyroid carcinoma: impact of change in WHO definition and molecular analysis. Endocr Pathol. 2019; 30:43–8.

43. Beninato T, Scognamiglio T, Kleiman DA, Uccelli A, Vaca D, Fahey TJ 3rd, et al. Ten percent tall cells confer the aggressive features of the tall cell variant of papillary thyroid carcinoma. Surgery. 2013; 154:1331–6.

44. Dettmer MS, Schmitt A, Steinert H, Capper D, Moch H, Komminoth P, et al. Tall cell papillary thyroid carcinoma: new diagnostic criteria and mutations in BRAF and TERT. Endocr Relat Cancer. 2015; 22:419–29.

45. Vuong HG, Long NP, Anh NH, Nghi TD, Hieu MV, Hung LP, et al. Papillary thyroid carcinoma with tall cell features is as aggressive as tall cell variant: a meta-analysis. Endocr Connect. 2018; 7:R286–93.

46. Bongers PJ, Kluijfhout WP, Verzijl R, Lustgarten M, Vermeer M, Goldstein DP, et al. Papillary thyroid cancers with focal tall cell change are as aggressive as tall cell variants and should not be considered as low-risk disease. Ann Surg Oncol. 2019; 26:2533–9.

47. Oh WJ, Lee YS, Cho U, Bae JS, Lee S, Kim MH, et al. Classic papillary thyroid carcinoma with tall cell features and tall cell variant have similar clinicopathologic features. Korean J Pathol. 2014; 48:201–8.

48. Rivera M, Ghossein RA, Schoder H, Gomez D, Larson SM, Tuttle RM. Histopathologic characterization of radioactive iodine-refractory fluorodeoxyglucose-positron emission tomography-positive thyroid carcinoma. Cancer. 2008; 113:48–56.

49. Silver CE, Owen RP, Rodrigo JP, Rinaldo A, Devaney KO, Ferlito A. Aggressive variants of papillary thyroid carcinoma. Head Neck. 2011; 33:1052–9.

50. Shi X, Liu R, Basolo F, Giannini R, Shen X, Teng D, et al. Differential clinicopathological risk and prognosis of major papillary thyroid cancer variants. J Clin Endocrinol Metab. 2016; 101:264–74.

51. Villar-Taibo R, Peteiro-Gonzalez D, Cabezas-Agricola JM, Aliyev E, Barreiro-Morandeira F, Ruiz-Ponte C, et al. Aggressiveness of the tall cell variant of papillary thyroid carcinoma is independent of the tumor size and patient age. Oncol Lett. 2017; 13:3501–7.

52. Enriquez ML, Baloch ZW, Montone KT, Zhang PJ, LiVolsi VA. CDX2 expression in columnar cell variant of papillary thyroid carcinoma. Am J Clin Pathol. 2012; 137:722–6.

53. Sujoy V, Pinto A, Nose V. Columnar cell variant of papillary thyroid carcinoma: a study of 10 cases with emphasis on CDX2 expression. Thyroid. 2013; 23:714–9.

54. Yunta PJ, Ponce JL, Prieto M, Merino F, Sancho-Fornos S. The importance of a tumor capsule in columnar cell thyroid carcinoma: a report of two cases and review of the literature. Thyroid. 1999; 9:815–9.

55. Huang WT, Yang SF, Wang SL, Chan HM, Chai CY. Encapsulated columnar-cell carcinoma of the thyroid: a case report. Kaohsiung J Med Sci. 2005; 21:241–4.

56. Chen JH, Faquin WC, Lloyd RV, Nose V. Clinicopathological and molecular characterization of nine cases of columnar cell variant of papillary thyroid carcinoma. Mod Pathol. 2011; 24:739–49.

57. Ieni A, Barresi V, Cardia R, Licata L, Di Bari F, Benvenga S, et al. The micropapillary/hobnail variant of papillary thyroid carcinoma: a review of series described in the literature compared to a series from one southern Italy pathology institution. Rev Endocr Metab Disord. 2016; 17:521–7.

58. Kakudo K, Tang W, Ito Y, Mori I, Nakamura Y, Miyauchi A. Papillary carcinoma of the thyroid in Japan: subclassification of common type and identification of low risk group. J Clin Pathol. 2004; 57:1041–6.

59. Bai Y, Kakudo K, Li Y, Liu Z, Ozaki T, Ito Y, et al. Subclassification of non-solid-type papillary thyroid carcinoma identification of high-risk group in common type. Cancer Sci. 2008; 99:1908–15.

60. Bai Y, Kakudo K, Nakamura M, Ozaki T, Li Y, Liu Z, et al. Loss of cellular polarity/cohesiveness in the invasive front of papillary thyroid carcinoma and periostin expression. Cancer Lett. 2009; 281:188–95.

61. Asioli S, Erickson LA, Sebo TJ, Zhang J, Jin L, Thompson GB, et al. Papillary thyroid carcinoma with prominent hobnail features: a new aggressive variant of moderately differentiated papillary carcinoma: a clinicopathologic, immunohistochemical, and molecular study of eight cases. Am J Surg Pathol. 2010; 34:44–52.

62. Liu Z, Kakudo K, Bai Y, Li Y, Ozaki T, Miyauchi A, et al. Loss of cellular polarity/cohesiveness in the invasive front of papillary thyroid carcinoma, a novel predictor for lymph node metastasis; possible morphological indicator of epithelial mesenchymal transition. J Clin Pathol. 2011; 64:325–9.

63. Asioli S, Erickson LA, Righi A, Lloyd RV. Papillary thyroid carcinoma with hobnail features: histopathologic criteria to predict aggressive behavior. Hum Pathol. 2013; 44:320–8.

64. Ambrosi F, Righi A, Ricci C, Erickson LA, Lloyd RV, Asioli S. Hobnail variant of papillary thyroid carcinoma: a literature review. Endocr Pathol. 2017; 28:293–301.

65. Lee YS, Kim Y, Jeon S, Bae JS, Jung SL, Jung CK. Cytologic, clinicopathologic, and molecular features of papillary thyroid carcinoma with prominent hobnail features: 10 case reports and systematic literature review. Int J Clin Exp Pathol. 2015; 8:7988–97.
66. Teng L, Deng W, Lu J, Zhang J, Ren X, Duan H, et al. Hobnail variant of papillary thyroid carcinoma: molecular profiling and comparison to classical papillary thyroid carcinoma, poorly differentiated thyroid carcinoma and anaplastic thyroid carcinoma. Oncotarget. 2017; 8:22023–33.

67. Lubitz CC, Economopoulos KP, Pawlak AC, Lynch K, Dias-Santagata D, Faquin WC, et al. Hobnail variant of papillary thyroid carcinoma: an institutional case series and molecular profile. Thyroid. 2014; 24:958–65.

68. Wong KS, Chen TY, Higgins SE, Howitt BE, Lorch JH, Alexander EK, et al. A potential diagnostic pitfall for hobnail variant of papillary thyroid carcinoma. Histopathology. 2020; 76:707–13.

69. Volante M, Collini P, Nikiforov YE, Sakamoto A, Kakudo K, Katoh R, et al. Poorly differentiated thyroid carcinoma: the Turin proposal for the use of uniform diagnostic criteria and an algorithmic diagnostic approach. Am J Surg Pathol. 2007; 31:1256–64.

70. Collini P, Mattavelli F, Pellegrinelli A, Barisella M, Ferrari A, Massimino M. Papillary carcinoma of the thyroid gland of childhood and adolescence: morphologic subtypes, biologic behavior and prognosis: a clinicopathologic study of 42 sporadic cases treated at a single institution during a 30-year period. Am J Surg Pathol. 2006; 30:1420–6.
71. LiVolsi VA, Abrosimov AA, Bogdanova T, Fadda G, Hunt JL, Ito M, et al. The Chernobyl thyroid cancer experience: pathology. Clin Oncol (R Coll Radiol). 2011; 23:261–7.

72. Nikiforov YE, Erickson LA, Nikiforova MN, Caudill CM, Lloyd RV. Solid variant of papillary thyroid carcinoma: incidence, clinical-pathologic characteristics, molecular analysis, and biologic behavior. Am J Surg Pathol. 2001; 25:1478–84.
73. Tronko MD, Bogdanova TI, Komissarenko IV, Epstein OV, Oliynyk V, Kovalenko A, et al. Thyroid carcinoma in children and adolescents in Ukraine after the Chernobyl nuclear accident: statistical data and clinicomorphologic characteristics. Cancer. 1999; 86:149–56.

74. Ohashi R. Solid variant of papillary thyroid carcinoma: an under-recognized entity. Endocr J. 2020; 67:241–8.

75. Ohashi R, Kawahara K, Namimatsu S, Igarashi T, Sakatani T, Sugitani I, et al. Clinicopathological significance of a solid component in papillary thyroid carcinoma. Histopathology. 2017; 70:775–81.

76. Caplan RH, Wester S, Kisken AW. Diffuse sclerosing variant of papillary thyroid carcinoma: case report and review of the literature. Endocr Pract. 1997; 3:287–92.

77. Joung JY, Kim TH, Jeong DJ, Park SM, Cho YY, Jang HW, et al. Diffuse sclerosing variant of papillary thyroid carcinoma: major genetic alterations and prognostic implications. Histopathology. 2016; 69:45–53.

78. Sheu SY, Schwertheim S, Worm K, Grabellus F, Schmid KW. Diffuse sclerosing variant of papillary thyroid carcinoma: lack of BRAF mutation but occurrence of RET/PTC rearrangements. Mod Pathol. 2007; 20:779–87.

79. Pillai S, Gopalan V, Smith RA, Lam AK. Diffuse sclerosing variant of papillary thyroid carcinoma: an update of its clinicopathological features and molecular biology. Crit Rev Oncol Hematol. 2015; 94:64–73.

80. Thompson LD, Wieneke JA, Heffess CS. Diffuse sclerosing variant of papillary thyroid carcinoma: a clinicopathologic and immunophenotypic analysis of 22 cases. Endocr Pathol. 2005; 16:331–48.

81. Koo JS, Hong S, Park CS. Diffuse sclerosing variant is a major subtype of papillary thyroid carcinoma in the young. Thyroid. 2009; 19:1225–31.

82. Fukushima M, Ito Y, Hirokawa M, Akasu H, Shimizu K, Miyauchi A. Clinicopathologic characteristics and prognosis of diffuse sclerosing variant of papillary thyroid carcinoma in Japan: an 18-year experience at a single institution. World J Surg. 2009; 33:958–62.

83. Malandrino P, Russo M, Regalbuto C, Pellegriti G, Moleti M, Caff A, et al. Outcome of the diffuse sclerosing variant of papillary thyroid cancer: a meta-analysis. Thyroid. 2016; 26:1285–92.

84. Kim HJ, Sung JY, Oh YL, Kim JH, Son YI, Min YK, et al. Association of vascular invasion with increased mortality in patients with minimally invasive follicular thyroid carcinoma but not widely invasive follicular thyroid carcinoma. Head Neck. 2014; 36:1695–700.

85. Xu B, Ghossein R. Encapsulated thyroid carcinoma of follicular cell origin. Endocr Pathol. 2015; 26:191–9.

86. O’Neill CJ, Vaughan L, Learoyd DL, Sidhu SB, Delbridge LW, Sywak MS. Management of follicular thyroid carcinoma should be individualised based on degree of capsular and vascular invasion. Eur J Surg Oncol. 2011; 37:181–5.

87. Cracolici V, Ritterhouse LL, Segal JP, Puranik R, Wanjari P, Kadri S, et al. Follicular thyroid neoplasms: comparison of clinicopathologic and molecular features of atypical adenomas and follicular thyroid carcinomas. Am J Surg Pathol. 2020; 44:881–92.
88. Maximo V, Sobrinho-Simoes M. Mitochondrial DNA ‘common’ deletion in Hurthle cell lesions of the thyroid. J Pathol. 2000; 192:561–2.
89. Bishop JA, Wu G, Tufano RP, Westra WH. Histological patterns of locoregional recurrence in Hürthle cell carcinoma of the thyroid gland. Thyroid. 2012; 22:690–4.

90. Haq M, Harmer C. Differentiated thyroid carcinoma with distant metastases at presentation: prognostic factors and outcome. Clin Endocrinol (Oxf). 2005; 63:87–93.

91. Gasparre G, Porcelli AM, Bonora E, Pennisi LF, Toller M, Iommarini L, et al. Disruptive mitochondrial DNA mutations in complex I subunits are markers of oncocytic phenotype in thyroid tumors. Proc Natl Acad Sci U S A. 2007; 104:9001–6.

92. Maximo V, Soares P, Lima J, Cameselle-Teijeiro J, Sobrinho-Simoes M. Mitochondrial DNA somatic mutations (point mutations and large deletions) and mitochondrial DNA variants in human thyroid pathology: a study with emphasis on Hurthle cell tumors. Am J Pathol. 2002; 160:1857–65.
93. Chindris AM, Casler JD, Bernet VJ, Rivera M, Thomas C, Kachergus JM, et al. Clinical and molecular features of Hurthle cell carcinoma of the thyroid. J Clin Endocrinol Metab. 2015; 100:55–62.

94. Ganly I, Makarov V, Deraje S, Dong Y, Reznik E, Seshan V, et al. Integrated genomic analysis of Hurthle cell cancer reveals oncogenic drivers, recurrent mitochondrial mutations, and unique chromosomal landscapes. Cancer Cell. 2018; 34:256–70.

95. Jung CK, Kim Y, Jeon S, Jo K, Lee S, Bae JS. Clinical utility of EZH1 mutations in the diagnosis of follicular-patterned thyroid tumors. Hum Pathol. 2018; 81:9–17.

96. Sakamoto A, Kasai N, Sugano H. Poorly differentiated carcinoma of the thyroid: a clinicopathologic entity for a high-risk group of papillary and follicular carcinomas. Cancer. 1983; 52:1849–55.

97. Hiltzik D, Carlson DL, Tuttle RM, Chuai S, Ishill N, Shaha A, et al. Poorly differentiated thyroid carcinomas defined on the basis of mitosis and necrosis: a clinicopathologic study of 58 patients. Cancer. 2006; 106:1286–95.

98. Asioli S, Erickson LA, Righi A, Jin L, Volante M, Jenkins S, et al. Poorly differentiated carcinoma of the thyroid: validation of the Turin proposal and analysis of IMP3 expression. Mod Pathol. 2010; 23:1269–78.

99. Bai S, Baloch ZW, Samulski TD, Montone KT, LiVolsi VA. Poorly differentiated oncocytic (Hürthle cell) follicular carcinoma: an institutional experience. Endocr Pathol. 2015; 26:164–9.

100. Ziad el A, Ruchala M, Breborowicz J, Gembicki M, Sowinski J, Grzymislawski M. Immunoexpression of TTF-1 and Ki-67 in a coexistent anaplastic and follicular thyroid cancer with rare long-life surviving. Folia Histochem Cytobiol. 2008; 46:461–4.
101. Kakudo K, Wakasa T, Ohta Y, Yane K, Ito Y, Yamashita H. Prognostic classification of thyroid follicular cell tumors using Ki-67 labeling index: risk stratification of thyroid follicular cell carcinomas. Endocr J. 2015; 62:1–12.

102. Deeken-Draisey A, Yang GY, Gao J, Alexiev BA. Anaplastic thyroid carcinoma: an epidemiologic, histologic, immunohistochemical, and molecular single-institution study. Hum Pathol. 2018; 82:140–8.

103. Dettmer M, Schmitt A, Steinert H, Haldemann A, Meili A, Moch H, et al. Poorly differentiated thyroid carcinomas: how much poorly differentiated is needed? Am J Surg Pathol. 2011; 35:1866–72.
104. Wong KS, Lorch JH, Alexander EK, Marqusee E, Cho NL, Nehs MA, et al. Prognostic significance of extent of invasion in poorly differentiated thyroid carcinoma. Thyroid. 2019; 29:1255–61.

105. Ibrahimpasic T, Ghossein R, Shah JP, Ganly I. Poorly differentiated carcinoma of the thyroid gland: current status and future prospects. Thyroid. 2019; 29:311–21.

106. Bournaud C, Descotes F, Decaussin-Petrucci M, Berthiller J, de la Fouchardiere C, Giraudet AL, et al. TERT promoter mutations identify a high-risk group in metastasis-free advanced thyroid carcinoma. Eur J Cancer. 2019; 108:41–9.

108. Keutgen XM, Sadowski SM, Kebebew E. Management of anaplastic thyroid cancer. Gland Surg. 2015; 4:44–51.
109. Kuhn E, Ragazzi M, Ciarrocchi A, Torricelli F, de Biase D, Zanetti E, et al. Angiosarcoma and anaplastic carcinoma of the thyroid are two distinct entities: a morphologic, immunohistochemical, and genetic study. Mod Pathol. 2019; 32:787–98.

110. Talbott I, Wakely PE Jr. Undifferentiated (anaplastic) thyroid carcinoma: practical immunohistochemistry and cytologic look-alikes. Semin Diagn Pathol. 2015; 32:305–10.

111. Bishop JA, Sharma R, Westra WH. PAX8 immunostaining of anaplastic thyroid carcinoma: a reliable means of discerning thyroid origin for undifferentiated tumors of the head and neck. Hum Pathol. 2011; 42:1873–7.

112. Lai WA, Hang JF, Liu CY, Bai Y, Liu Z, Gu H, et al. PAX8 expression in anaplastic thyroid carcinoma is less than those reported in early studies: a multi-institutional study of 182 cases using the monoclonal antibody MRQ-50. Virchows Arch. 2020; 476:431–7.

113. Nakazawa T, Kondo T, Vuong HG, Odate T, Kawai M, Tahara I, et al. High expression of CD10 in anaplastic thyroid carcinomas. Histopathology. 2018; 73:492–9.

114. Oh EJ, Bychkov A, Cho H, Kim TM, Bae JS, Lim DJ, et al. Prognostic implications of CD10 and CD15 expression in papillary thyroid carcinoma. Cancers (Basel). 2020; 12:1413.

115. Xu B, Fuchs T, Dogan S, Landa I, Katabi N, Fagin JA, et al. Dissecting anaplastic thyroid carcinoma: a comprehensive clinical, histologic, immunophenotypic, and molecular study of 360 cases. Thyroid. 2020; 30:1505–17.

116. Leeman-Neill RJ, Kelly LM, Liu P, Brenner AV, Little MP, Bogdanova TI, et al. ETV6-NTRK3 is a common chromosomal rearrangement in radiation-associated thyroid cancer. Cancer. 2014; 120:799–807.

117. Dogan S, Wang L, Ptashkin RN, Dawson RR, Shah JP, Sherman EJ, et al. Mammary analog secretory carcinoma of the thyroid gland: a primary thyroid adenocarcinoma harboring ETV6-NTRK3 fusion. Mod Pathol. 2016; 29:985–95.

118. Stevens TM, Kovalovsky AO, Velosa C, Shi Q, Dai Q, Owen RP, et al. Mammary analog secretory carcinoma, low-grade salivary duct carcinoma, and mimickers: a comparative study. Mod Pathol. 2015; 28:1084–100.

Fig. 1
Noninvasive follicular thyroid neoplasm with papillary-like nuclear features. (A) Gross photo showing an encapsulated solid nodule confined to the thyroid gland. (B) Microscopically, the tumor shows a follicular growth pattern. Some follicular cells reveal mild nuclear enlargement, nuclear membrane irregularity, and a pale chromatin pattern which correspond to a nuclear score of 3 (H&E stain, ×400).
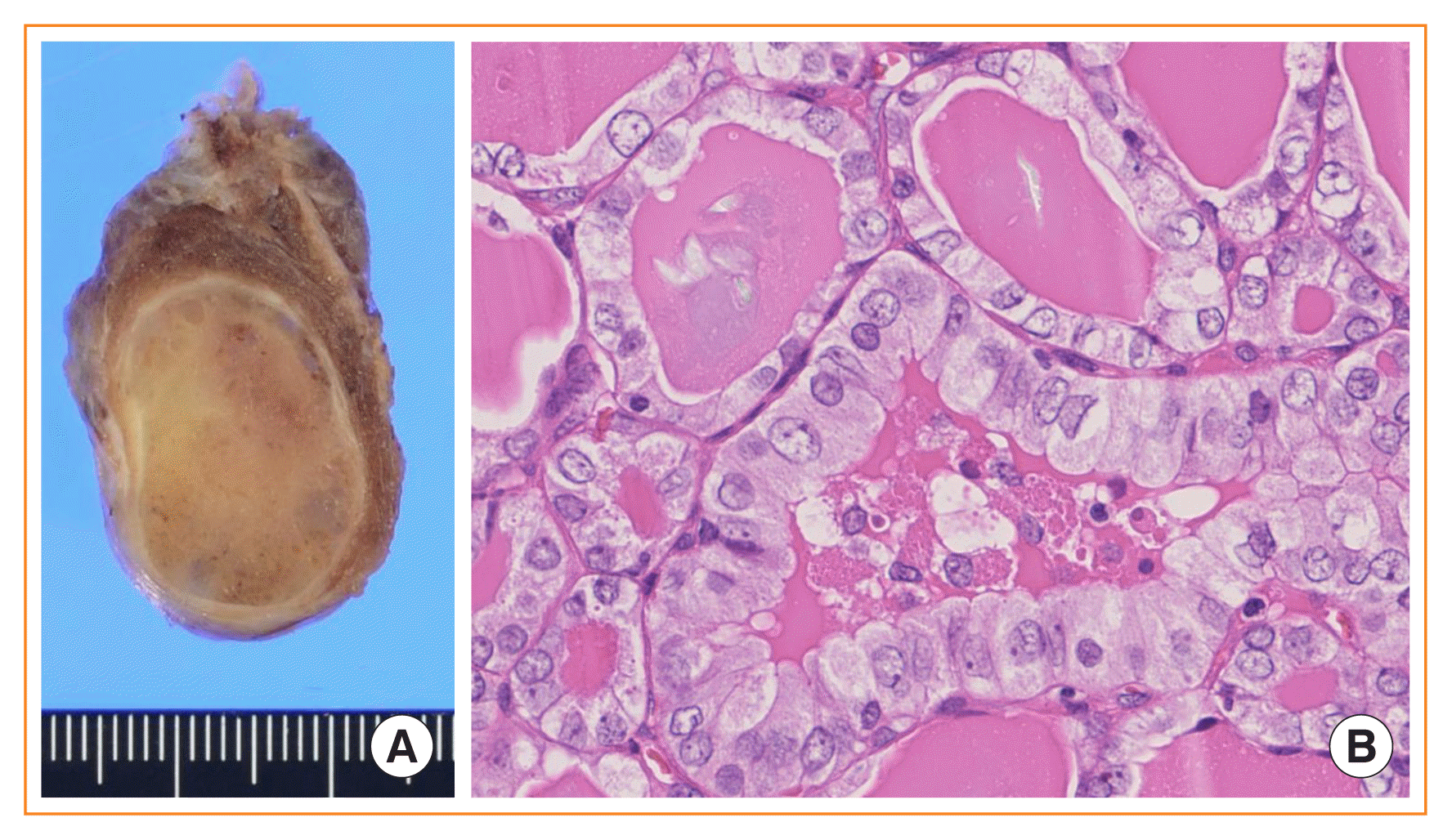
Fig. 2
Schematic explanation of encapsulated follicular patterned thyroid tumors. NIFTP, noninvasive follicular thyroid neoplasm with papillary-like nuclear features; FV-PTC, follicular variant papillary thyroid carcinoma; FT-UMP, follicular tumor of uncertain malignant potential; WDT-UMP, well-differentiated tumor of uncertain malignant potential; WDC-NOS, well differentiated carcinoma not otherwise specified.
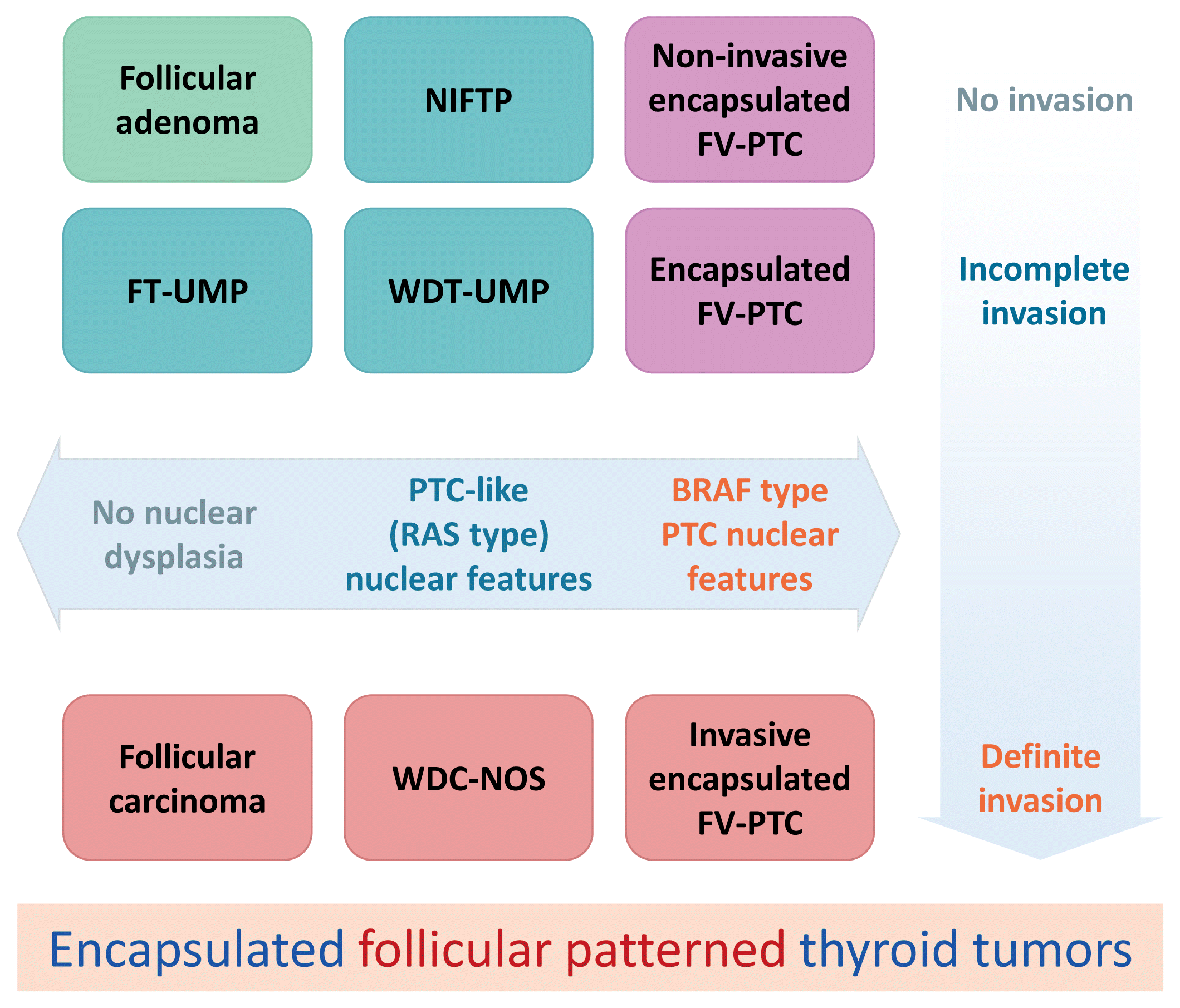
Fig. 3
Schematic explanation of encapsulated papillary patterned thyroid tumors. Noninvasive encapsulated papillary patterned thyroid tumors with papillary thyroid carcinoma (PTC)-like nuclear features were not defined in the fourth edition World Health Organization classification of tumors of endocrine organs [2]. Ohba et al. [27] proposed to name it noninvasive encapsulated papillary RAS-like thyroid tumor (NEPRAS). FT-UMP, follicular tumor of uncertain malignant potential; WDT-UMP, well-differentiated tumor of uncertain malignant potential; WDC-NOS, well-differentiated carcinoma not otherwise specified.
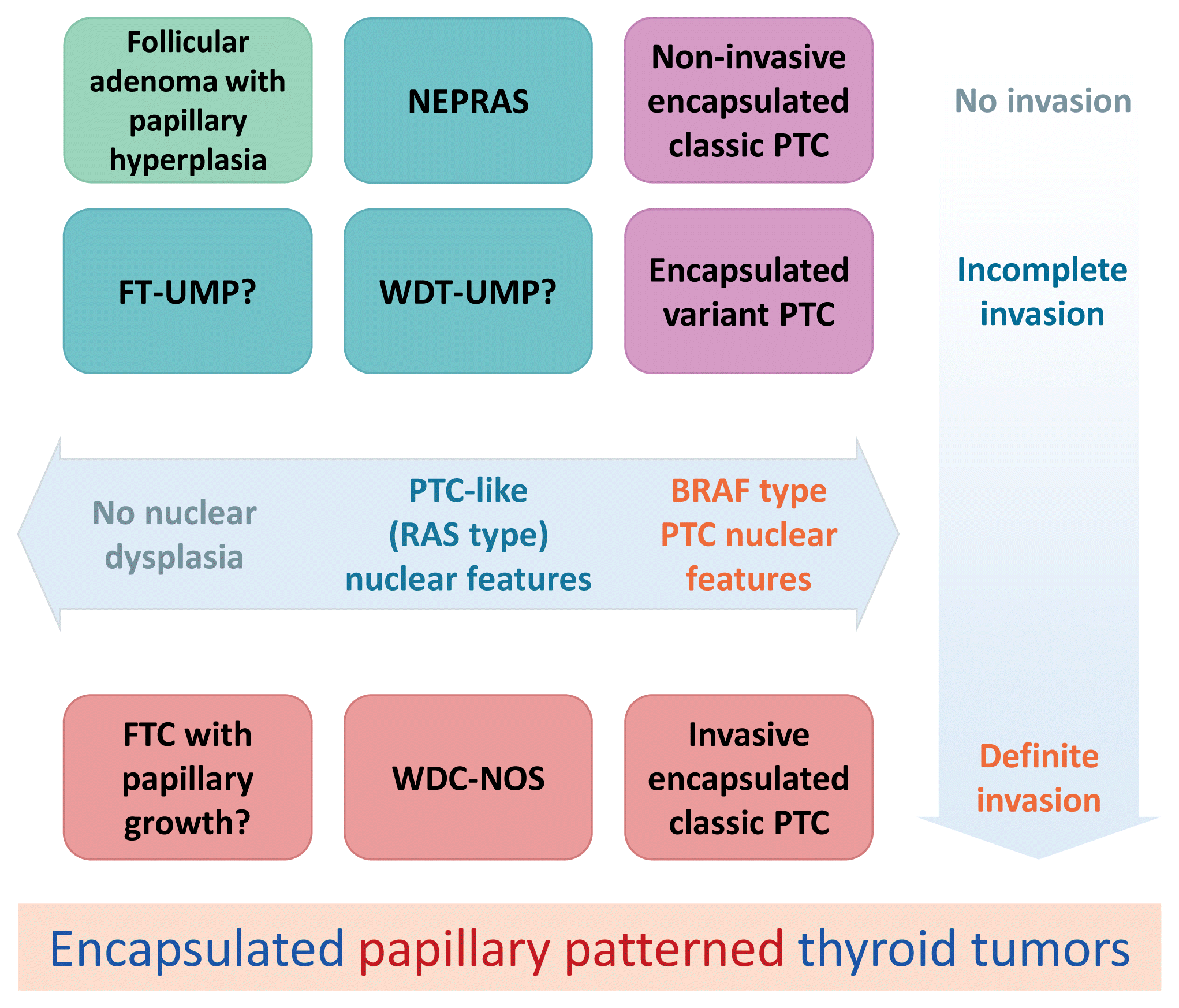
Fig. 4
Noninvasive encapsulated papillary RAS-like thyroid tumor (NEPRAS). (A) Low-power view of an encapsulated papillary patterned thyroid without capsular or vascular invasion (H&E stain, ×40). (B) Higher magnification reveals mild nuclear enlargement and nuclear membrane irregularity which correspond to a nuclear score of 2 (H&E stain, ×400). Ohba et al. [27] proposed to name this tumor NEPRAS.
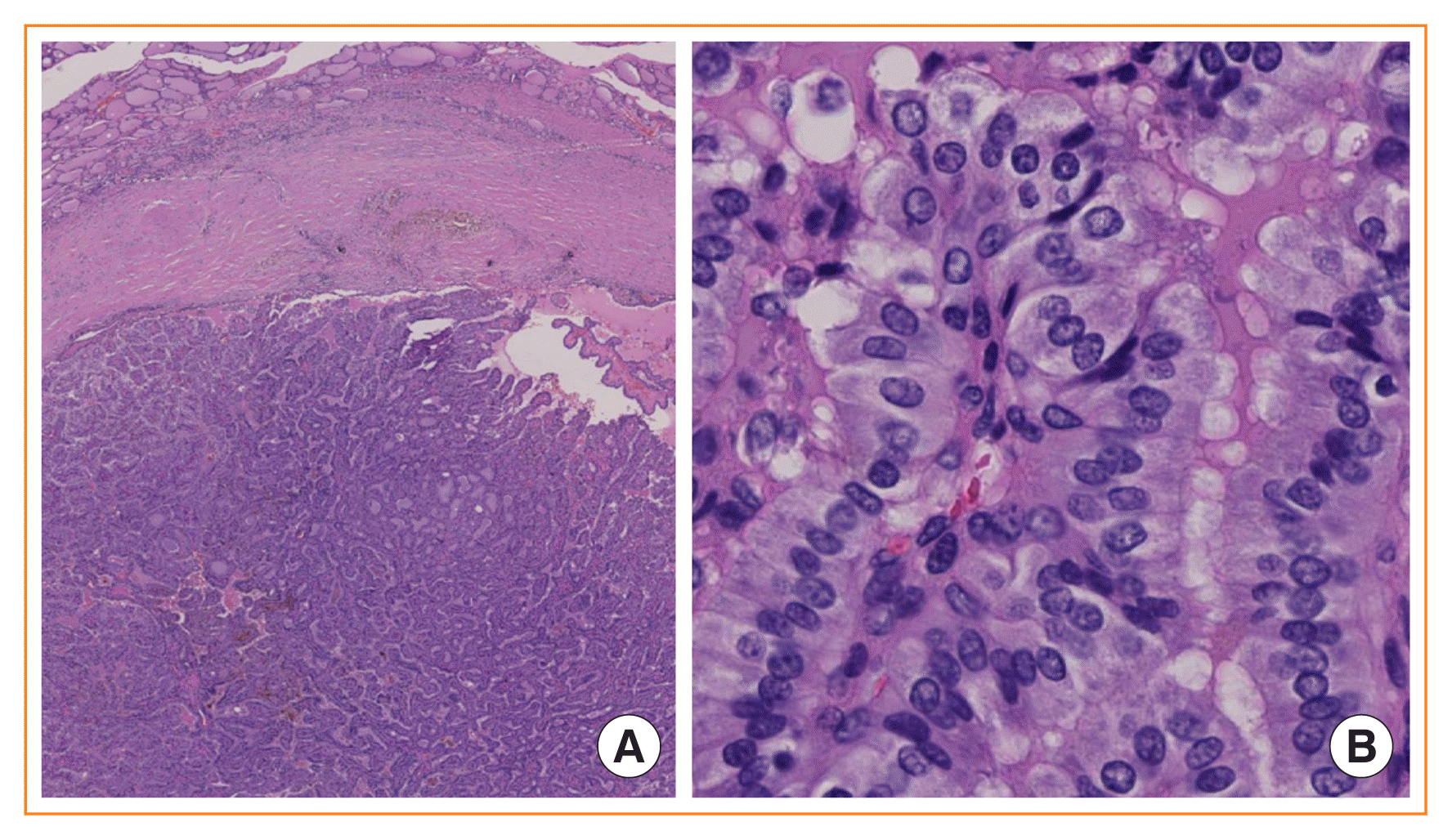
Fig. 5
Schematic explanation of how to classify four different noninvasive encapsulated thyroid tumors including encapsulated papillary thyroid carcinoma (PTC), noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP), noninvasive encapsulated papillary RAS-like thyroid tumor (NEPRAS), and follicular adenoma. NS, nuclear score.
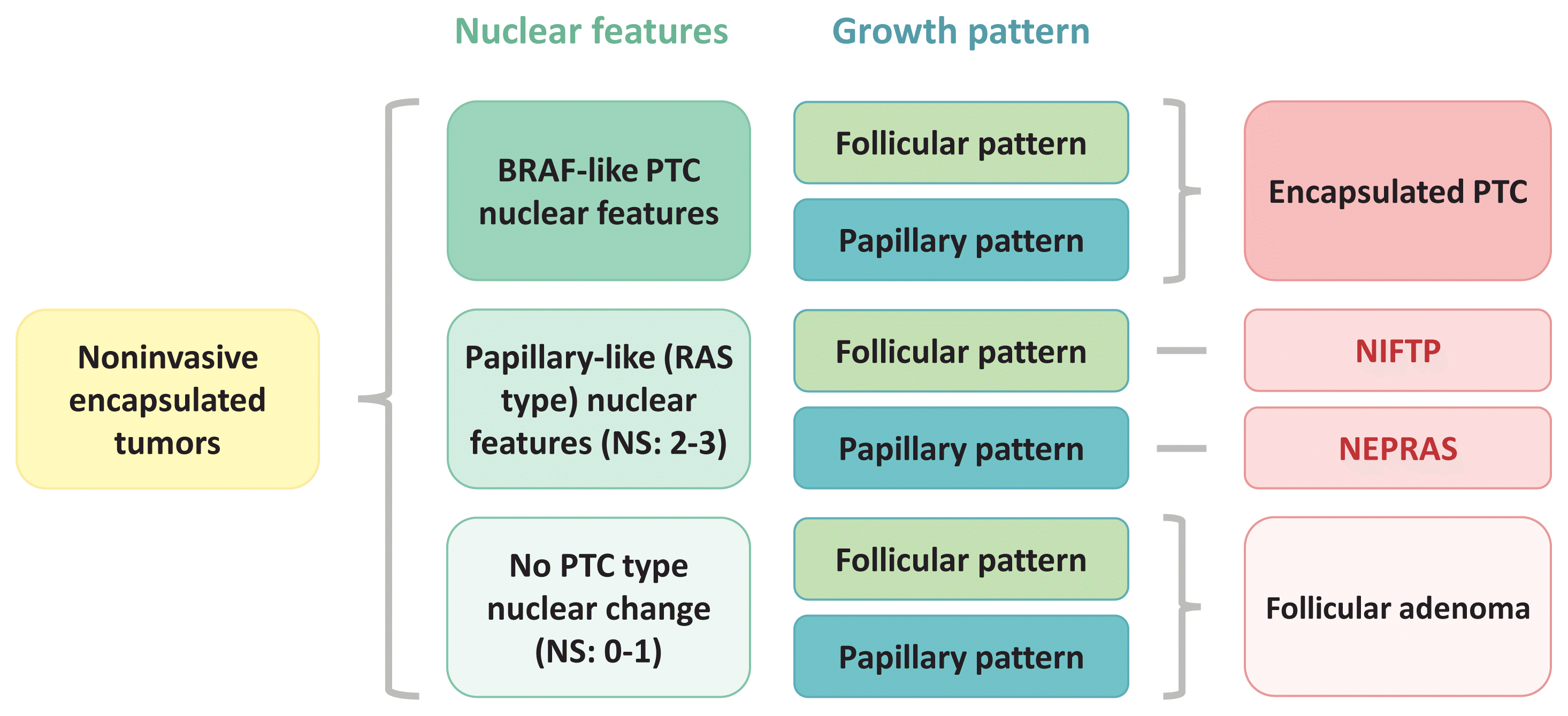
Fig. 6
Hyalinizing trabecular tumor. (A) Tumor cells are arranged in a trabecular pattern and show a spindle shape, abundant cytoplasm, and papillary thyroid carcinoma-like nuclear features (H&E stain, ×400). (B) Fine needle aspiration cytology shows intranuclear pseudoinclusions (arrows) (H&E stain, ×400).
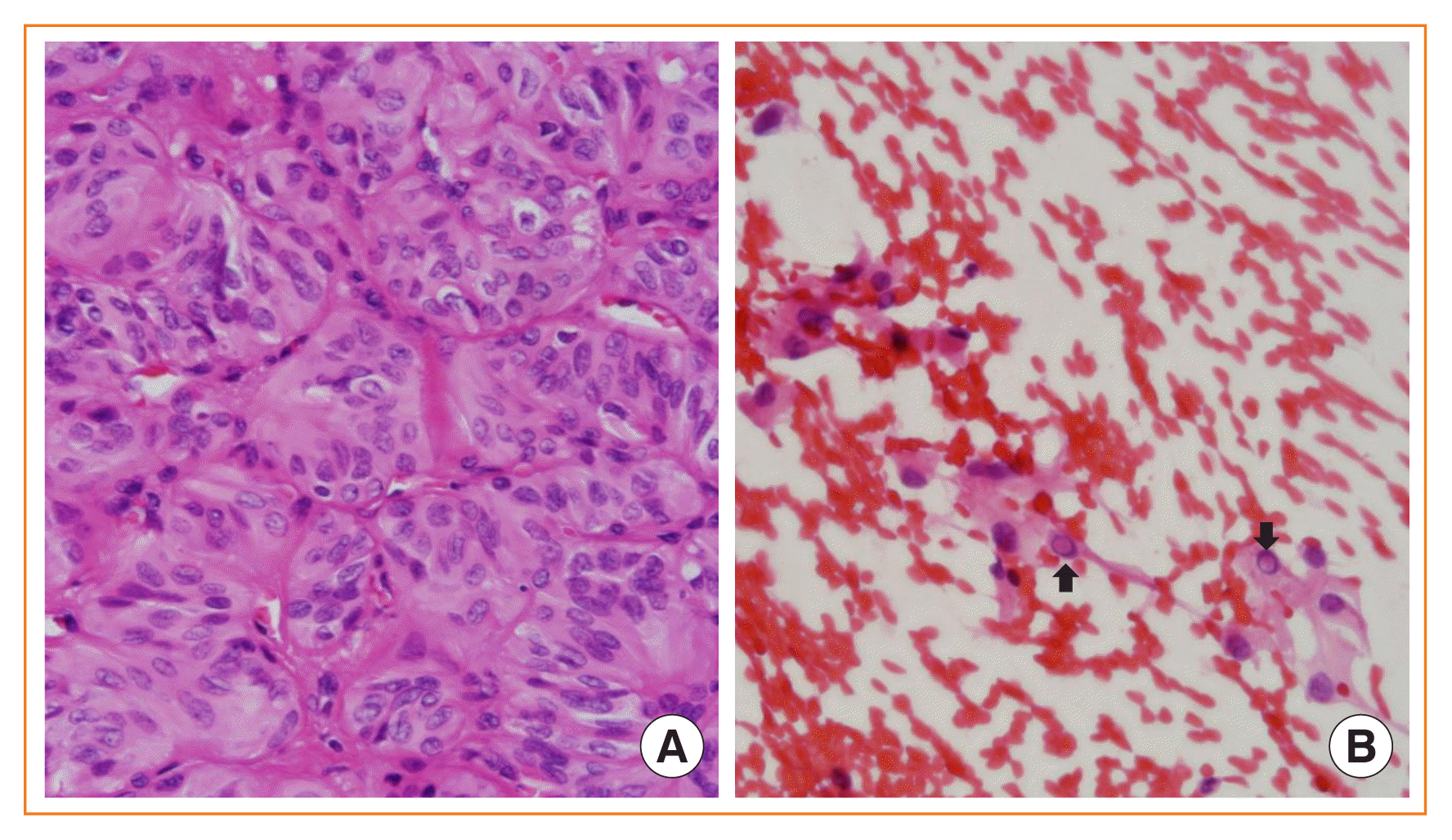
Fig. 7
Tall cell variant of papillary thyroid carcinoma (PTC) mixed with classic papillary PTC. (A) The region of tall cells comprises more than 30% of the tumor (H&E stain, ×40). (B) Tall cells are arranged in a “tram-track” parallel pattern and are more than twice as tall as they are wide (H&E stain, ×400).
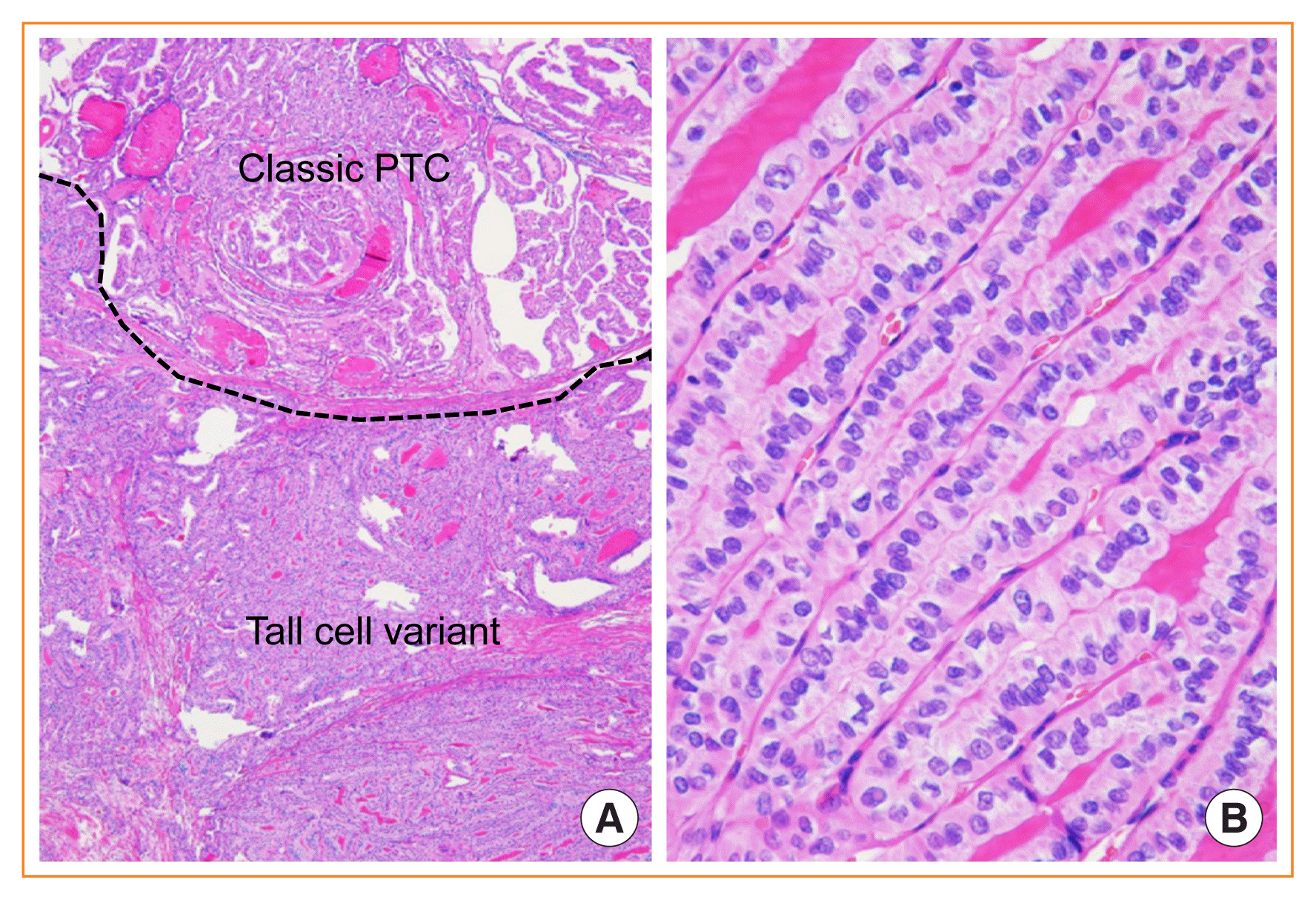
Fig. 8
Columnar cell variant of papillary thyroid carcinoma (PTC). (A) Papillary and glandular architecture lined by columnar cells showing nuclear stratification and lacking characteristic nuclear features of PTC (H&E stain, ×400). (B) Columnar cells resemble secretory-type endometrium having supranuclear cytoplasmic vacuoles (H&E stain, ×400).
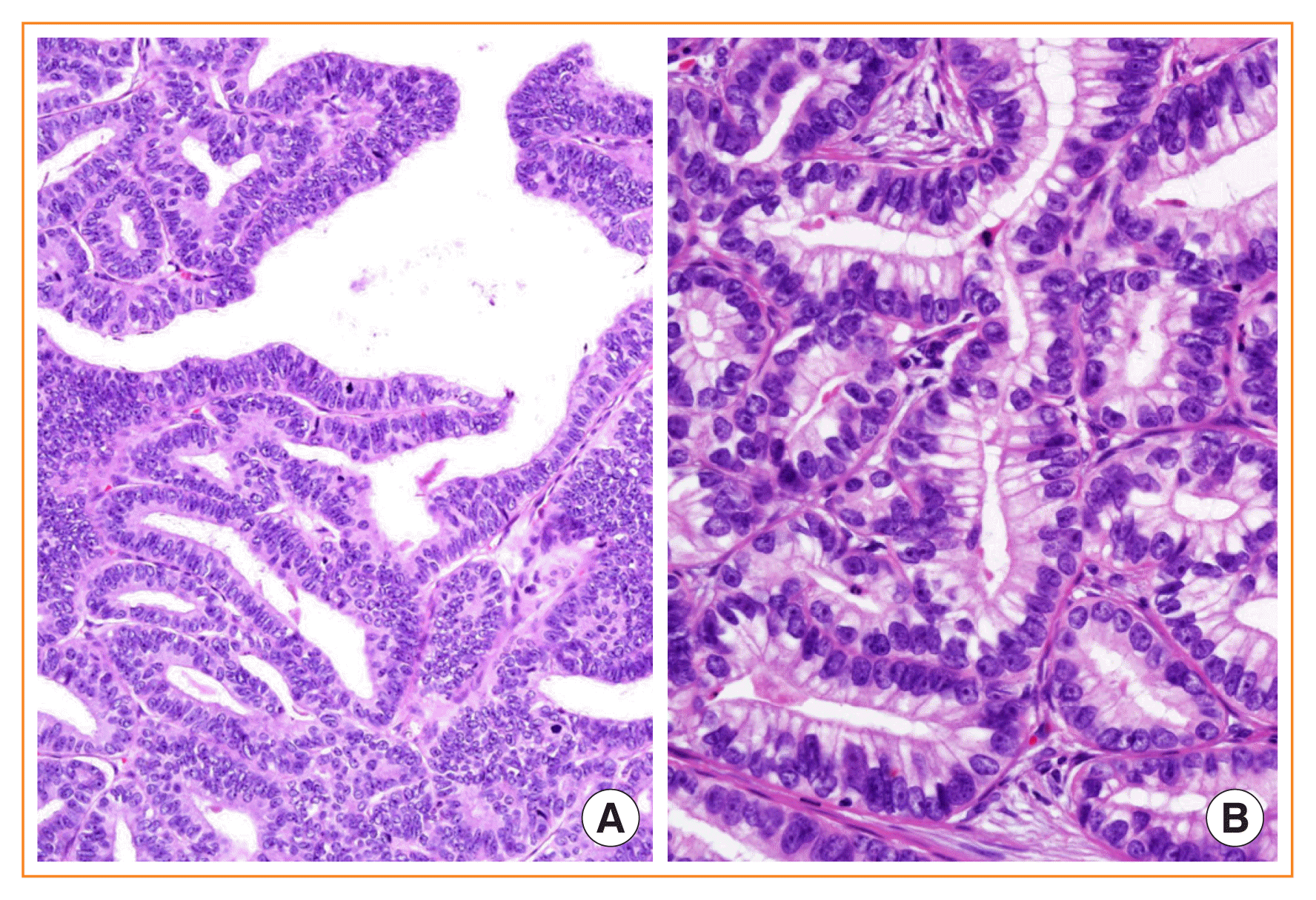
Fig. 9
Hobnail variant of papillary thyroid carcinoma (PTC) and mimicker. (A) True hobnail variant (H&E stain, ×400). (B) Hobnail-like morphology is often seen in classic PTC with cystic changes (H&E stain, ×400).
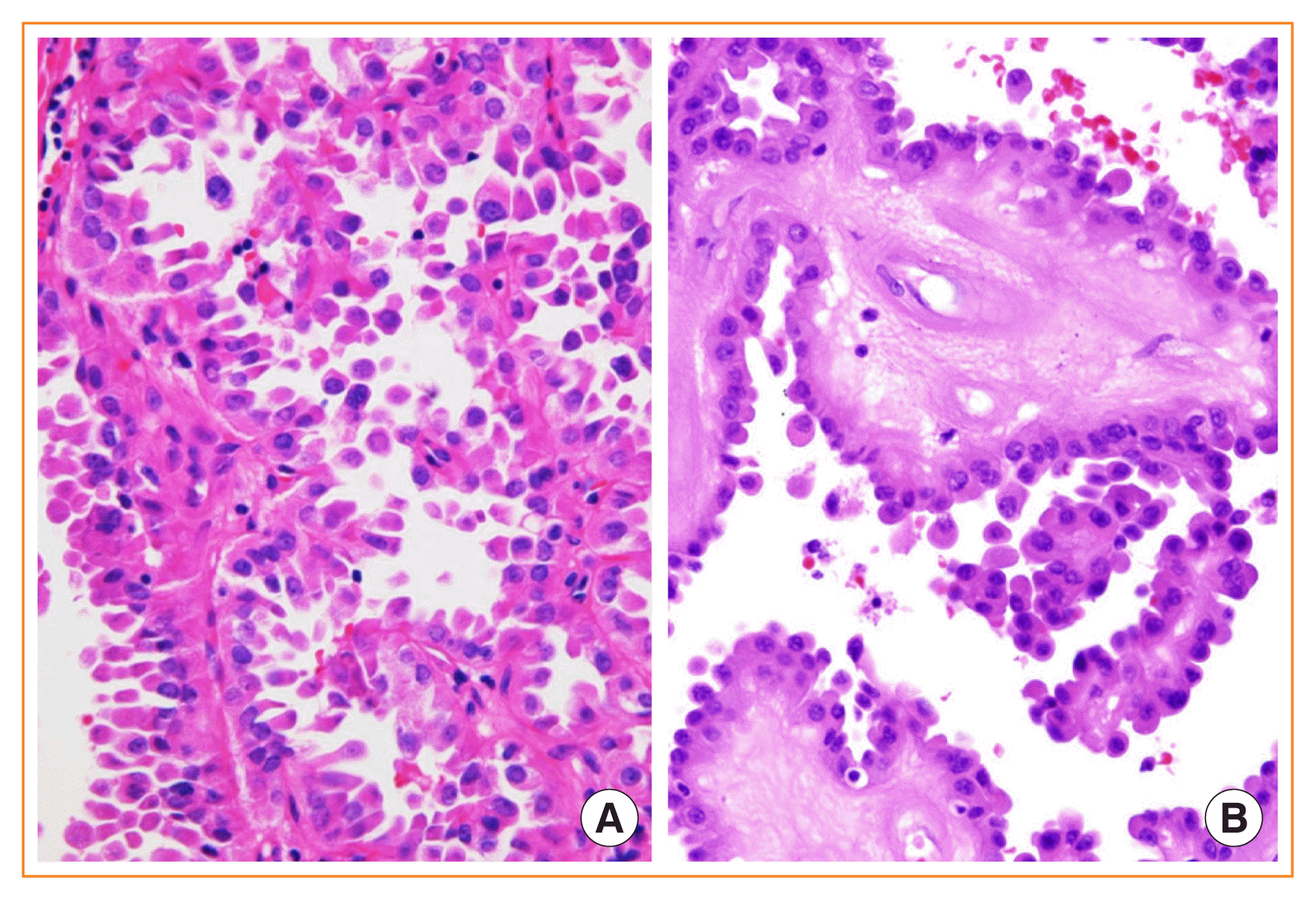
Fig. 10
Follicular thyroid carcinoma (FTC). (A) Minimally invasive FTC showing capsular invasion only (H&E stain, ×16). (B) A focus of vascular invasion found in an encapsulated angioinvasive FTC (H&E stain, ×253).
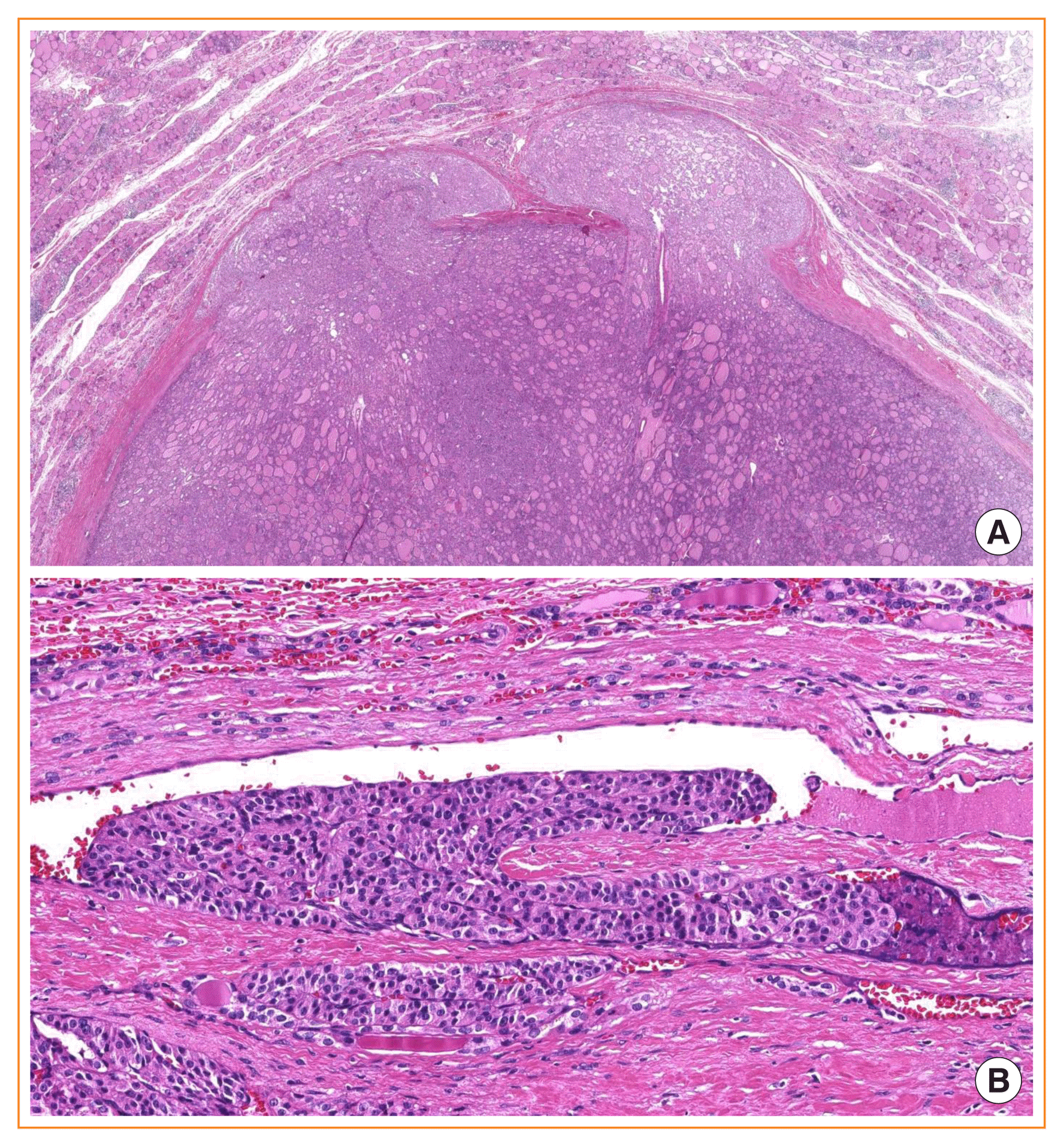
Fig. 11
Classification of encapsulated thyroid tumors of follicular cell origin according to growth pattern and molecular profiles. Noninvasive encapsulated papillary RAS-like thyroid tumor (NEPRAS) is not currently included as a distinct entity of World Health Organization classification. PTC, papillary thyroid carcinoma; HA, Hürthle cell adenoma; HCC, Hürthle cell carcinoma; FA, follicular adenoma; FTC, follicular thyroid carcinoma; NIFTP, noninvasive follicular thyroid neoplasm with papillary-like nuclear features; iEFVPTC, invasive encapsulated follicular variant of papillary thyroid carcinoma.
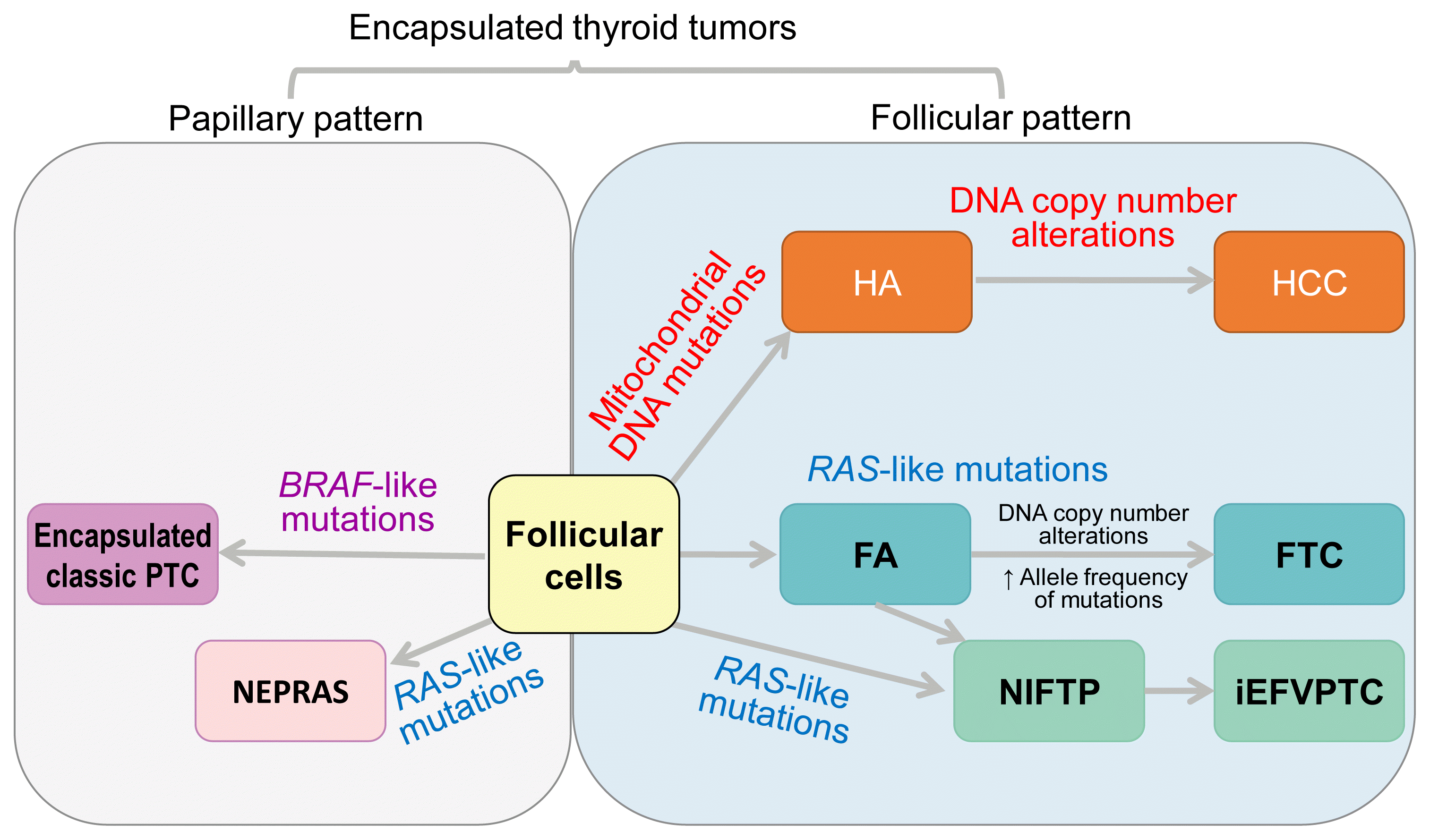
Fig. 12
Well-differentiated thyroid carcinoma with high-grade features. Papillary thyroid carcinoma showing necrosis (A, H&E stain, ×100) and high proliferative activity with a Ki-67 rate of 18% (B, H&E stain, ×400). Follicular thyroid carcinoma with necrosis (C, H&E stain, ×100) and high mitotic figure (D, H&E stain, ×400). Three mitotic figures (arrows) are noted in one high-power field.
Fig. 13
Anaplastic thyroid carcinoma (ATC) associated with papillary thyroid carcinoma (PTC). (A) Left area is ATC and right is PTC (H&E stain, ×100). (B) CD10 immunostain shows a diffuse and strong positivity in ATC (left) and is negative in PTC (right) (×100). (C) High-power view image shows pleomorphic tumor cells (H&E stain, ×400). (D) PAX8 immunostain shows a focal positivity (×400).

Table 1
Revisions to the Classification and Nomenclature of Thyroid Tumors
Table 2
The 2017 World Health Organization Classification of Thyroid Tumors
Table 3
Histologic Criteria for the Diagnosis of Variants of Papillary Thyroid Carcinoma
Table 4
Two Systems of Classification of Thyroid Tumors by Ki-67 Labeling Index
| WHO classification [2] | Prognostic classification proposed by Kakudo et al. [101] | ||
|---|---|---|---|
| Tumors | Ki-67 | Tumors | Ki-67 |
| Benign | |||
| Normal thyroid follicular cells | <3% | Follicular adenoma | <3% |
| Borderline tumorsa, encapsulated tumors | |||
| WDT-UMP, FT-UMP, NIFTP, NEPRAS, noninvasive encapsulated PTC, minimally invasive FTC (capsular invasion only) | <3% | ||
| Borderline tumors, non-encapsulated (infiltrative) tumors | |||
| Well-differentiated thyroid carcinoma | <10% | Papillary microcarcinoma | <3% |
| Malignant tumors (invasive carcinoma, >1 cm) | |||
| Low risk invasive | ≤5% | ||
| Moderate risk | 5%–10% | ||
| Poorly differentiated carcinoma | 10%–30% | High risk | 10%–30% |
| Anaplastic thyroid carcinoma | > 30% | Anaplastic thyroid carcinoma | >30% |
WHO, World Health Organization; WDT-UMP, well-differentiated tumor of uncertain malignant potential; FT-UMP, follicular tumor of uncertain malignant potential; NIFTP, noninvasive follicular thyroid neoplasm with papillary like nuclear features; NEPRAS, noninvasive encapsulated papillary RAS-like thyroid tumor; PTC, papillary thyroid carcinoma; FTC, follicular thyroid carcinoma.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download