Abstract
Background
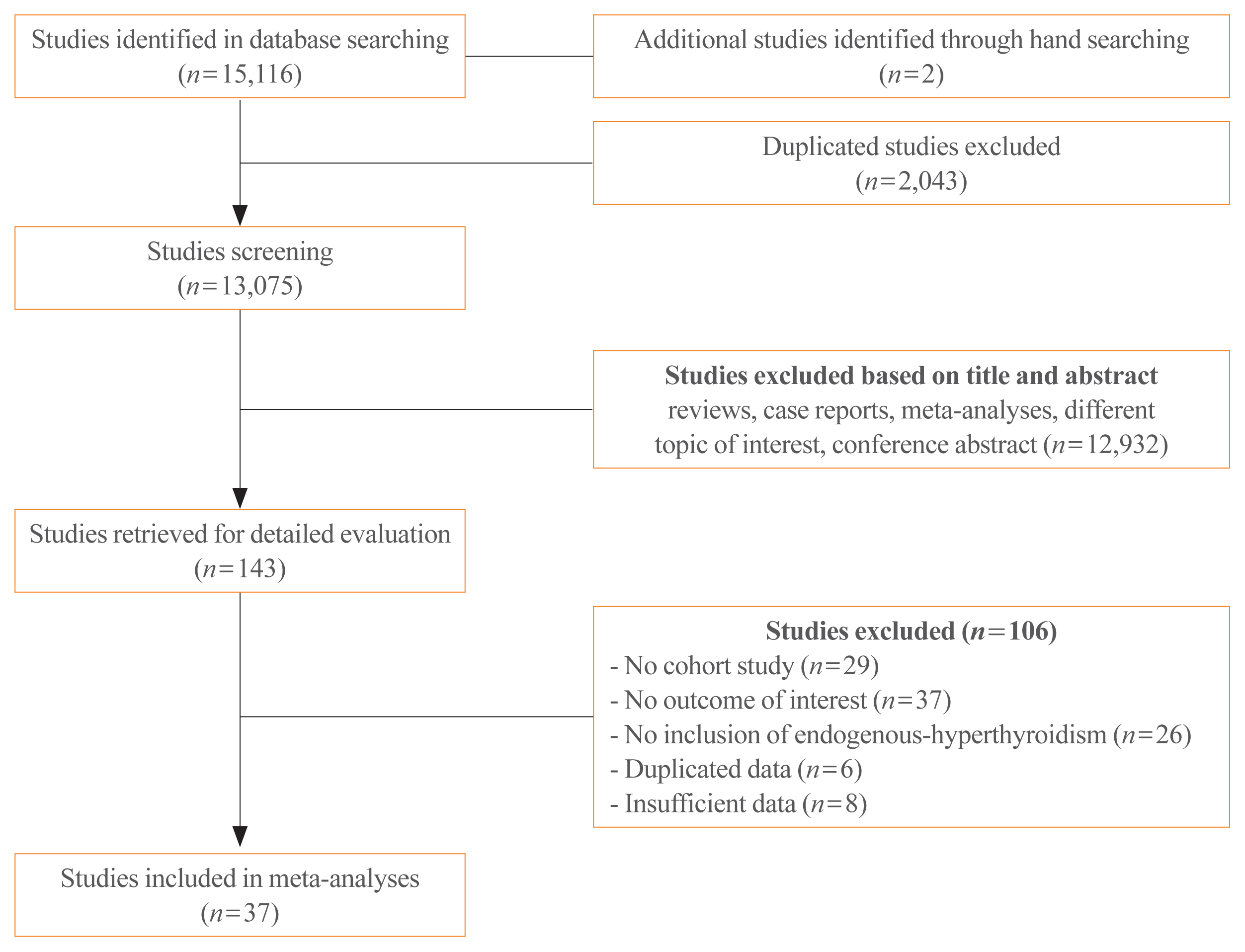
Methods
Results
Supplementary Data
Supplemental Table S2.
Supplemental Table S3.
Supplemental Table S4.
ACKNOWLEDGMENTS
REFERENCES
Fig. 2
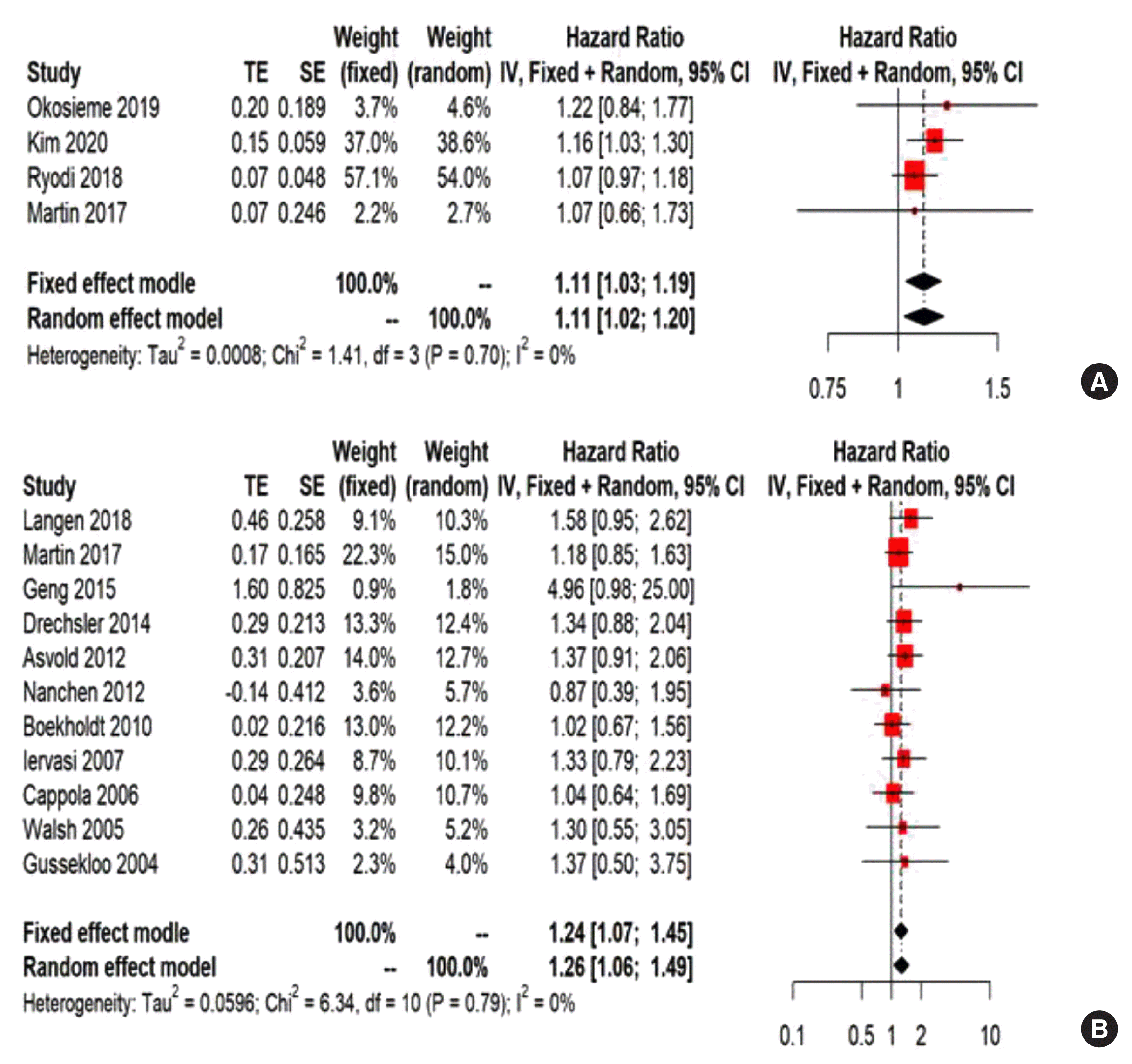
Fig. 3
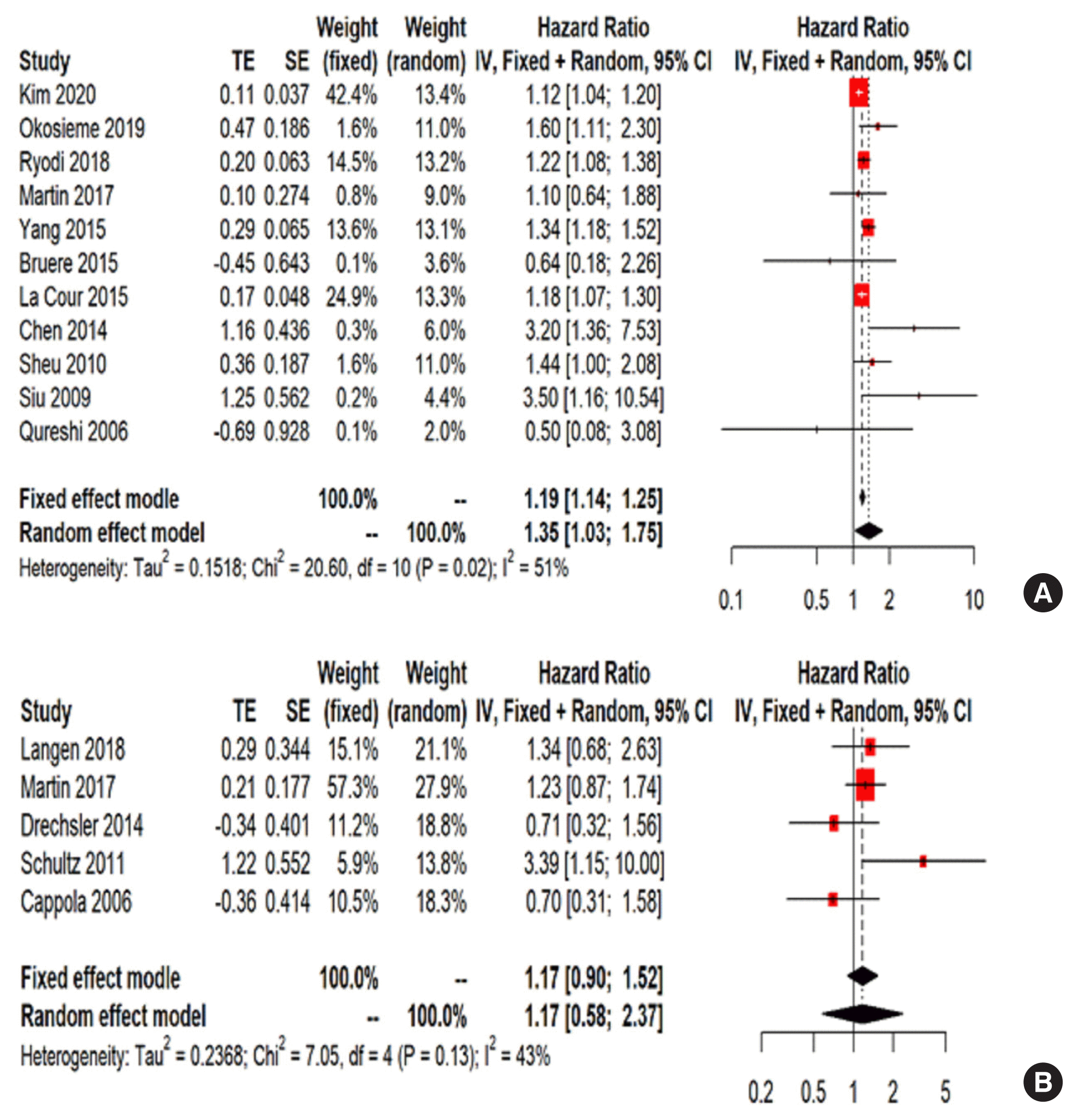
Fig. 4
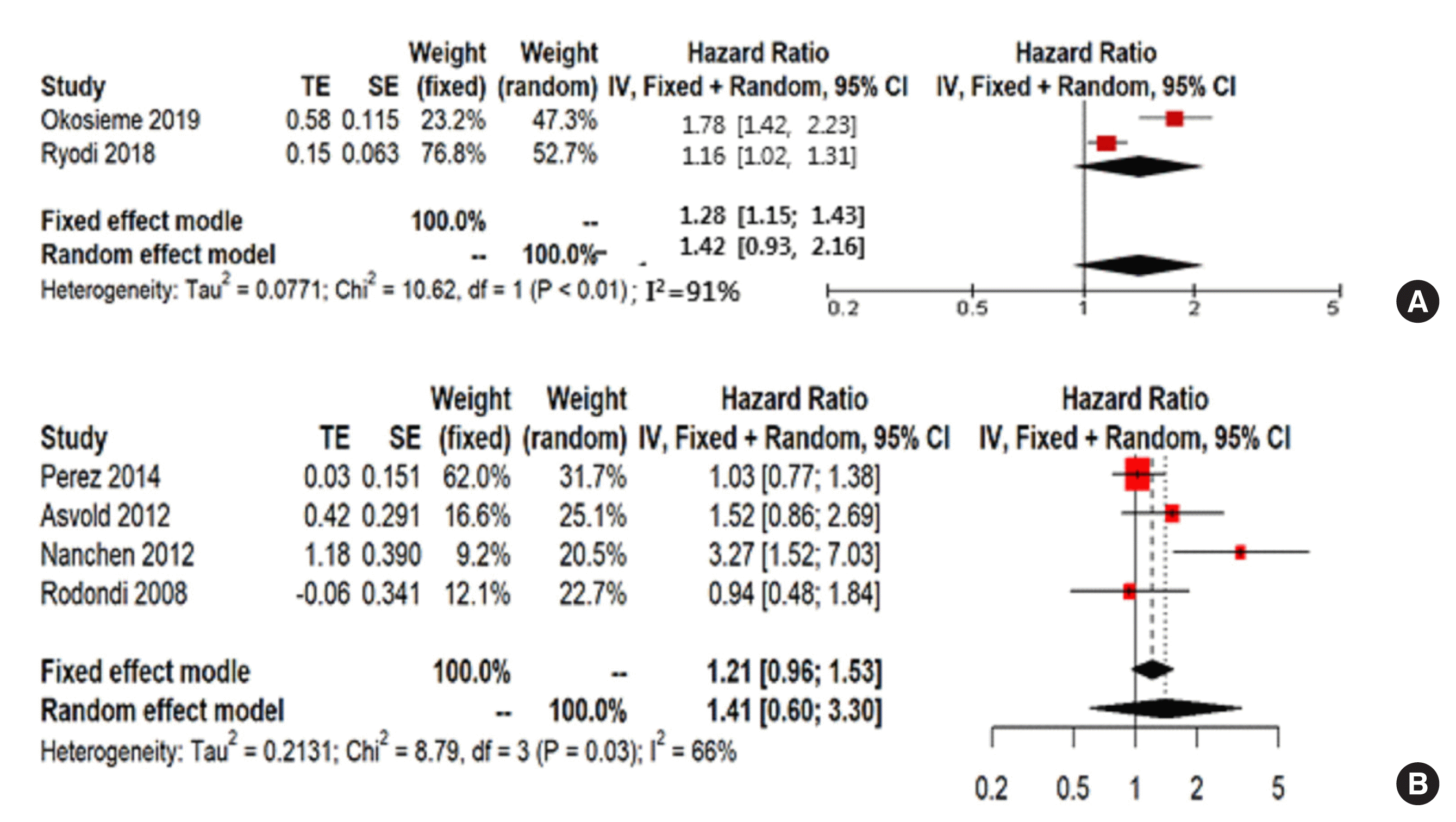
Fig. 5
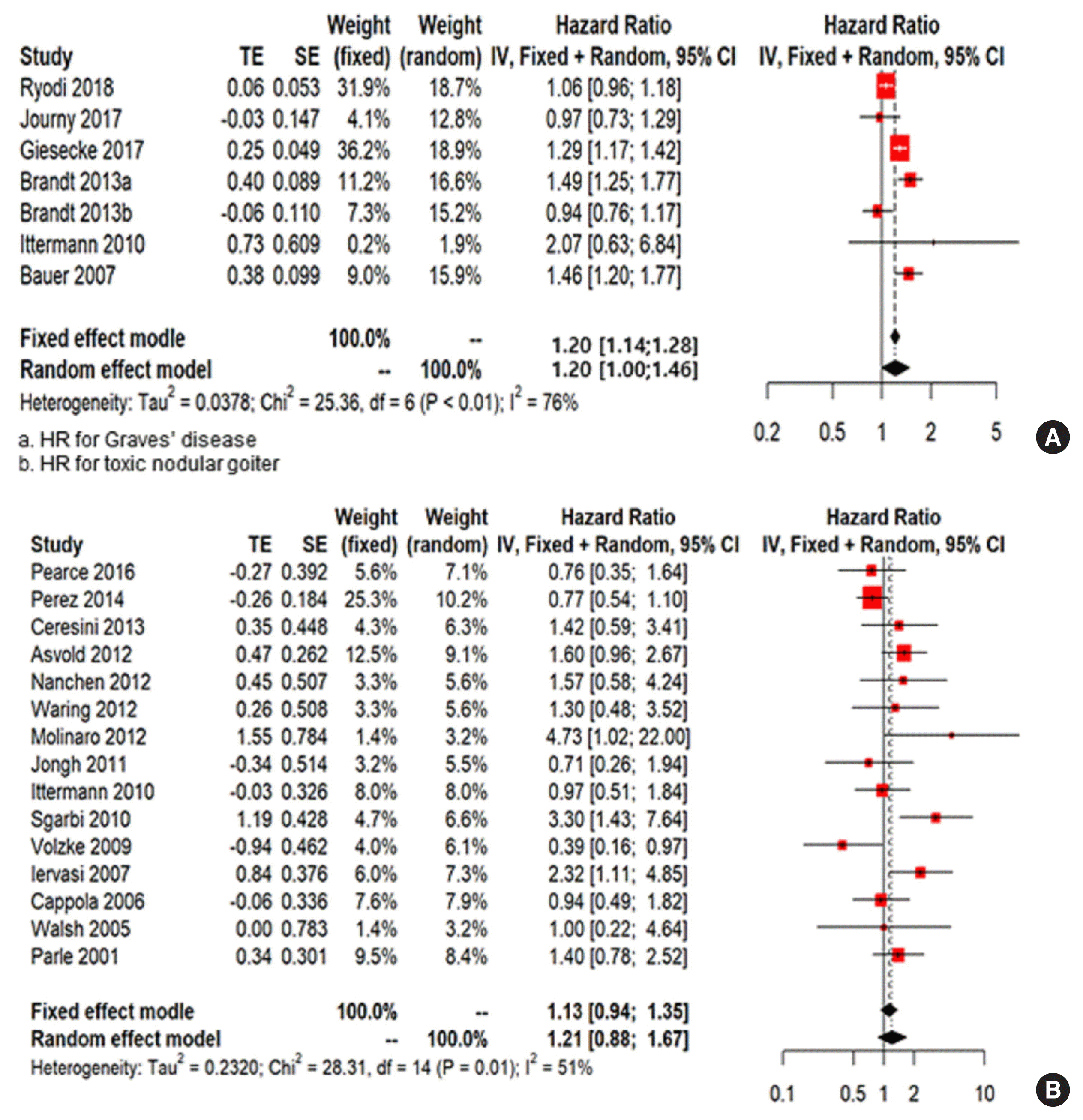
Table 1
| Study | Country | Study population | Classification of hyperthyroidism | Mean or median age | No. of total subjects | No. of hyperthyroid subjects | CV outcome |
|---|---|---|---|---|---|---|---|
| Kim et al. (2020) [26] | Korea | Community dwelling | Overt |
Overt: 48.5 Control: 48.5 |
1,239,441 | 59,021 | IHD and stroke |
| Okosieme et al. (2019) [32] | England | Community dwelling | Overt |
Overt: 48±16 Control: 48±16 |
20,945 | 4,189 | IHD, stroke, and HF |
| Langen et al. (2018) [28] | Finland | Community dwelling | Subclinical |
Subclinical: 56.8±16.5 Control: 50.5±13.8 |
5,211 | 108 | IHD and stroke |
| Ryodi et al. (2018) [17] | Finland | Community dwelling | Overt | 49 (35–63) | 24,580 | 6,148 | IHD, stroke, HF, and CV mortality |
| Journy et al. (2017) [25] | United States | Community dwelling | Overt |
Overt: <60 years Control: <60 years |
75,076 | 1,501 | CV mortality |
| Giesecke et al. (2017) [21] | Sweden | Community dwelling | Overt |
Overt: 61.3 Control: 49.1 |
15,924 | 12,239 | CV mortality |
| Martin et al. (2017) [29] | United States | Community dwelling | Overt, subclinical |
Overt: 56.7±5.8 Subclinical: 56.2±5.7 Control: 56.5±5.7 |
11,359 |
Overt: 206 Subclinical: 378 |
IHD and stroke |
| Pearce et al. (2016) [33] | United Kingdom | Community dwelling | Subclinical | 85.5±0.4 | 643 | 19 | CV mortality |
| Bruere et al. (2015) [9] | France | High CVD risk (A-fib patients) | Overt |
Overt: 71±14 Control: 68±13 |
8,962 | 141 | Stroke |
| Geng et al. (2015) [20] | China | High CVD risk (type 2 diabetes) | Subclinical |
Subclinical: 57.5±14.7 Control: 58.8±13.5 |
1,115 | 74 | IHD |
| la Cour et al. (2015) [27] | Denmark | Community dwelling | Overt |
Overt: 61.9±14.3 Control: 61.0±14.2 |
25,562 | 4,000 | Stroke |
| Yang et al. (2015) [42] | Taiwan | Community dwelling | Overt |
Overt: 40.9±14.3 Control: 41.3±14.5 |
68,462 | 16,808 | Stroke |
| Chen et al. (2014) [13] | China | High CVD risk (A-fib patients) | Overt |
Overt: 55.1±1.7 Control: 59.4±1.1 |
269 | 62 | Stroke |
| Drechsler et al. (2014) [16] | Germany | High CVD risk (diabetic hemodialysis) | Subclinical |
Subclinical: 66.9±7.9 Control: 65.3±8.5 |
1,000 | 137 | IHD and stroke |
| Perez et al. (2014) [34] | Europe, multicenter | High CVD risk (heart failure) | Subclinical |
Subclinical: 72.9±6.2 Control: 72.6±7.1 |
4,987 | 175 | HF and CV mortality |
| Brandt et al. (2013) [8] | Denmark | Community dwelling | Overt |
Graves’ disease: 55 (18–96) Toxic nodular goiter: 62 (18–96) |
10,760 | 2,152 | CV mortality |
| Ceresini et al. (2013) [11] | Italy | Community dwelling | Subclinical | >65 years | 951 | 83 | CV mortality |
| Asvold et al. (2012) [5] | Norway | Community dwelling | Subclinical |
Male: 53 (46–65) Female: 54 (47–67) |
26,707 | 524 | IHD and HF |
| Nanchen et al. (2012) [31] | Europe, multicenter | High CVD risk (CVD patients) | Subclinical |
Subclinical: 75.3±3.1 Control: 75.3±3.4 |
5,316 | 71 | IHD, HF, and CV mortality |
| Waring et al. (2012) [41] | United States | Community dwelling | Subclinical |
Subclinical: 74.1 Control: 73.6 |
1,587 | 41 | CV mortality |
| Molinaro et al. (2012) [30] | Italy | High CVD risk | Subclinical |
Subclinical: 69.3 (65–73) Control: 65 (64–66) |
1,026 | 23 | CV mortality |
| de Jongh et al. (2011) [15] | Netherland | Community dwelling | Subclinical |
Subclinical: 77.7±7.0 Control: 75.5±6.5 |
1,219 | 34 | CV mortality |
| Schultz et al. (2011) [36] | Denmark | High CVD risk (type 2 diabetes) | Subclinical |
Subclinical: 74±10 Control: 67.5±10.5 |
609 | 25 | Stroke and CV mortality |
| Boekholdt et al. (2010) [7] | United Kingdom | Community dwelling | Subclinical |
Subclinical: 59±10 Control: 58±9 |
11,554 | 216 | IHD |
| Ittermann et al. (2010) [24] | German | Community dwelling | Overt, subclinical |
Subclinical: 61 (48–69) Overt: NA Control: 48 (35–62) |
3,651 |
Subclinical: 243 Overt: 27 |
CV mortality |
| Sheu et al. (2010) [38] | Taiwan | Community dwelling | Overt |
Overt: 32.1±7.4 Control: 32.1±7.5 |
28,584 | 3,176 | Stroke |
| Sgarbi et al. (2010) [37] | Brazil | Community dwelling | Subclinical |
Subclinical: 61.4±12.5 Control: 56.4±12.4 |
1,110 | 69 | CV mortality |
| Siu et al. (2009) [39] | Hong Kong | High CVD risk (A-fib patients) | Overt |
Overt: 64.7±1.3 Control: 64.7±1.1 |
480 | 160 | Stroke |
| Volzke et al. (2009) [40] | Germany | High CVD risk (IHD patients) | Subclinical |
Subclinical: 62.0±7.9 Control: 61.0±8.0 |
942 | 118 | CV mortality |
| Rodondi et al. (2008) [35] | United States | Community dwelling | Subclinical |
Subclinical: 73.8±6.9 Control: 72.5±5.5 |
3,044 | 44 | HF |
| Bauer et al. (2007) [6] | United States | Community dwelling | Overt |
Overt: 72.3±5.6 Control: 71.6± 5.3 |
9,449 | 891 | CV mortality |
| Iervasi et al. (2007) [23] | Italy | High CVD (IHD patients) | Subclinical |
Subclinical: 60.5 (59–62) Control: 59.9 (59–60) |
3,121 | 98 | CV mortality, IHD |
| Cappola et al. (2006) [10] | United States | Community dwelling | Subclinical |
Subclinical: 73.9±6.8 Control: 72.6±5.6 |
3,233 | 31 | IHD, stroke, and CV mortality |
| Qureshi et al. (2006) [44] | United States | Community dwelling | Overt | 48±14 | 5,269 | 34 | Stroke |
| Walsh et al. (2005) [46] | Australia | Community dwelling | Subclinical |
Subclinical: 51.3±14.9 Control: 49.2±17.0 |
2,108 | 37 | IHD and CV mortality |
| Gussekloo et al. (2004) [22] | Netherland | Community dwelling | Subclinical | >85 years | 558 | 19 | CV mortality, IHD |
| Parle et al. (2001) [43] | United Kingdom | Community dwelling | Subclinical |
Male 70.1 (69.6–70.6) Female 70.7 (70.2–71.4) |
1,191 | 71 | CV mortality |
Table 2
Table 3
Appendix
Appendix 1
Study protocol
Title: The association of overt and subclinical hyperthyroidism with the risk of cardiovascular events and cardiovascular mortality: Meta-analysis and systematic review of cohort studies.
Objectives: This study is conducted to evaluate the association of overt and subclinical hyperthyroidism with the risk of ischemic heart disease (IHD), stroke, heart failure, and cardiovascular mortality.
Protocol and registration: Methods of database search, study selection, data extraction, assessment of study quality and risk of bias, and statistical analysis are predefined in the protocol at the beginning of the study.
Reporting: This meta-analysis and systematic review was reported according to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) statement.
-
Eligible criteria
-
Study characteristics
-
Report characteristics
-
Inclusion and exclusion criteria
We only included cohort studies investigating the relationship of hyperthyroidism with cardiovascular events.
We included cohort studies reporting at least one cardiovascular outcome including ischemic heart disease, stroke, heart failure, and cardiovascular mortality.
We included cohort studies reporting cardiovascular outcome as hazard ratio (HR) with 95% confidence interval (CI).
We included cohort studies comparing the endogenous hyperthyroidism between euthyroid control group and the general population without hyperthyroidism.
In case of duplicates or extensions, we only included a study with the longer duration or more information.
We included full-text articles with no restriction of publication status.
We excluded studies evaluating the effect of exogenous hyperthyroidism
-
Information sources: We searched electronic databases of MEDLINE, Embase, and the website of OpenGrey.
-
Search strategy: Cohort studies regarding the association between hyperthyroidism and cardiovascular events were searched using following criteria.
-
MEDLINE
#1. “Thyroid diseases” OR “hyperthyroidism” OR “thyroid function tests” OR “thyrotropin” OR “thyroid hormones”
#2. “Myocardial ischemia” OR “heart failure” OR “stroke” OR “brain ischemia” OR “mortality” OR “death”
#3. #1 AND #2
#4. “Hyperthyroidism” OR “thyroid dysfunction” OR “thyrotoxicosis” OR “Graves’ disease” OR “thyroid function” OR “thyroid diseases”
#5. “Ischemic heart disease” OR “coronary heart disease” OR “coronary disease” OR “myocardial infarction” OR “stroke” OR “heart failure” OR “death” OR “mortality” OR “cardiovascular”
#6. #4 AND #5
-
EMBASE
#1. ‘Hyperthyroidism’ OR ‘thyroid dysfunction’ OR ‘thyrotoxicosis’ OR ‘Graves’ disease’ OR ‘thyroid function’ OR ‘thyroid diseases’
#2. ‘Ischemic heart disease’ OR ‘coronary heart disease’ OR ‘coronary disease’ OR ‘myocardial infarction’ OR ‘stroke’ OR ‘heart failure’ OR ‘death’ OR ‘mortality’ OR ‘cardiovascular’
#3. #1 AND #2
-
Website of OpenGrey
#1. “Hyperthyroidism” OR “thyroid dysfunction” OR “thyrotoxicosis” OR “thyroid function” OR “thyroid diseases”
#2. “Ischemic heart disease” OR “coronary heart disease” OR “coronary disease” OR “myocardial infarction” OR “stroke” OR “heart failure” OR “death” OR “mortality” OR “cardiovascular”
#3. #1 AND #2
-
Study selection: All identified records were evaluated for eligibility by two reviewers independently. We reviewed titles, abstracts, and full texts of the studies thoroughly. Any disagreements were resolved by a third reviewer.
-
Data extraction: Standardized data extraction was performed by two reviewers independently as follows. Any disagreements were resolved by a third reviewer.
First author
Publication year
Country
Number of study participants
Characteristics of study participants: mean or median age, classification of hyperthyroidism, underlying comorbidity including history of coronary, cerebral, or peripheral artery disease; heart failure; atrial fibrillation; diabetes mellitus; or chronic kidney disease and regional iodine intake (sufficient area vs. deficient area)
Cardiovascular outcome explored
HRs for each cardiovascular outcome
-
Assessment of study quality and risk bias: We assessed quality and risk of bias of included studies using the Newcastle-Ottawa Scale. Two reviewers independently evaluated each study based on following aspects of trials:
Selection: representativeness of the exposed cohort, selection of the non-exposed cohort, ascertainment of hyperthyroidism, demonstration that outcome of interest was not present at start of study
Comparability: comparability of cohorts based on the design or analysis
Outcome: assessment of outcome, adequacy of follow-up of cohorts
-
Data synthesis
Statistical analysis: We calculated pooled HRs with 95% CI for each cardiovascular outcome. We used both fixed and random-effects model to evaluate pooled HR.
Subgroup analysis: We performed subgroup analyses by age (<65 years, ≥65 years), CVD risk and regional iodine intake status (sufficient area vs. deficient area).
Identifying and measuring statistical heterogeneity: We used I2 statistic for measuring the degree of heterogeneity.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download