INTRODUCTION
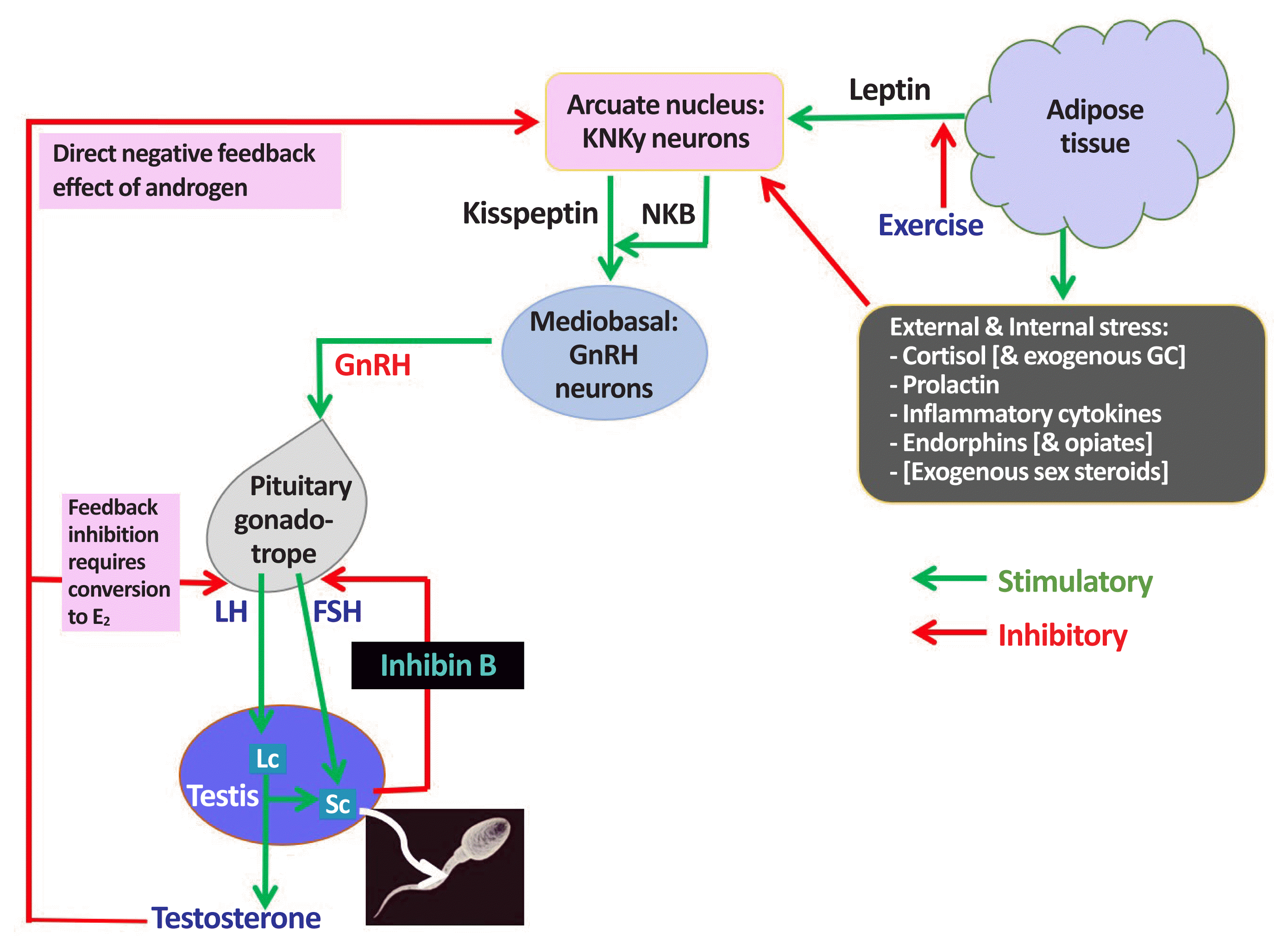 | Fig. 1Male hypothalamic-pituitary-gonadal (HPG) axis: homeostatic & environmental inputs: endocrine & paracrine actions of testosterone. KNDy, kisspeptin, neurokinin B, and dynorphin; NKB, neurokinin B; GC, glucocorticosteroid; GnRH, gonadotropin-releasing hormone; E2, oestradiol; FSH, follicle-stimulating hormone; LH, luteinizing hormone; Lc, Leydig cell; Sc, Sertoli cell. |
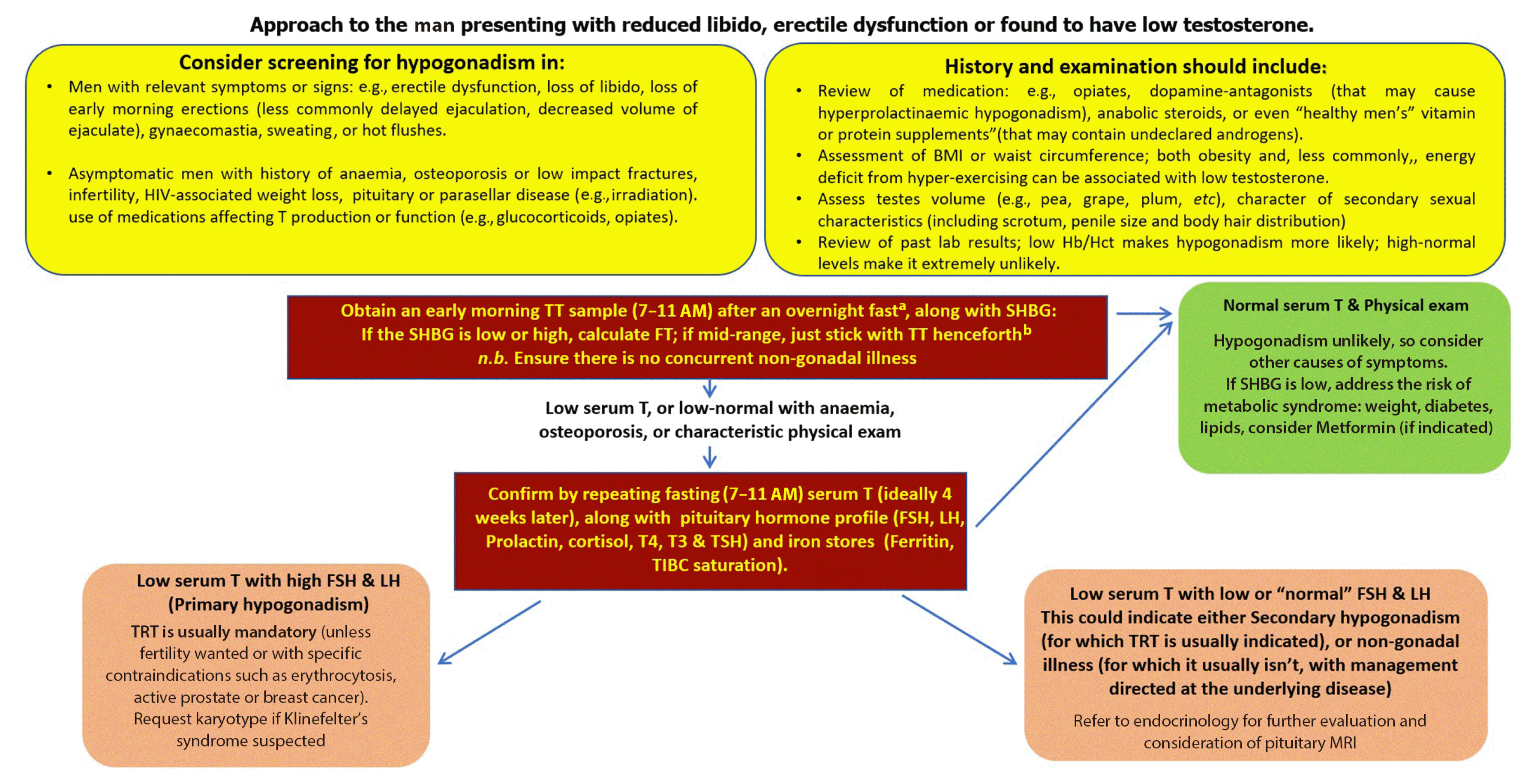 | Fig. 2An approach to low testosterone levels in primary care. HIV, human immunodeficiency virus; BMI, body mass index; Hb/Hct, hemoglobin/hematocrit; TT, total testosterone; SHBG, sex hormone binding globulin; FSH, follicle-stimulating hormone; LH, luteinizing hormone; T4, thyroxine; T3, triiodothyronine; TSH, thyroid stimulating hormone; TIBC, total iron binding capacity; TRT, testosterone replacement therapy; MRI, magnetic resonance imaging. aConsider your local laboratory reference range as measurements can be variable from laboratory to another, in shift workers measure within 3 hours of waking; bwww.issam.ch/freetesto.htm. |
DEFINING MH CLINICALLY AND BIOCHEMICALLY
WHO TO SCREEN FOR MH?
The CMAJ recommends screening (and potential treatment) of men with otherwise unexplained anemia, sarcopenia, treatment-refractory depression, chronic use of opioids or glucocorticoids, human immunodeficiency virus (HIV)-associated weight loss and erectile dysfunction (ED) resistant to first-line therapy.
The AUA suggests that measurement of serum T should be considered in men with a history of unexplained anemia, bone density loss, diabetes, exposure of the testes to chemotherapy or ionizing radiation, HIV/acquired immune deficiency syndrome (AIDS), chronic narcotic use, male infertility, pituitary dysfunction, and chronic glucocorticoid use.
The ISSM recommends screening for MH in men with obesity, T2DM, and metabolic syndrome, while the BSSM recommended screening in men with body mass index >30 kg/m2, waist circumference >102 cm and T2DM.
The ES recommends case detection for men deemed to be at increased risk of having hypogonadism and likely to benefit from T therapy, such as those with low libido, erectile dysfunction, infertility, HIV-associated weight loss, osteoporosis or low-trauma fracture, a history of anabolic steroids use, or using opioids or other drugs or substances that affect T production or metabolism.
SERUM T CUTOFF TO SUPPORT THE DIAGNOSIS OF MH
To rely on local laboratory normal ranges, or instead conform to a universal cut-off serum T value to define the lower limit of normality (LLN); if so, what that value should be?
Decrementing values for LLN should apply to correspondingly older men?
Diagnostic venepuncture should always be performed in the fasted state?
More than one sample is required for diagnostic purposes and, if so, what the minimum spacing between samples should be?
Serum T values should always be interpreted in the context of patients’ clinical presentations?
Obesity, diabetes, or metabolic syndrome are perceived as increasing the pre-test probability of MH, or conversely, acting to reduce the diagnostic specificity of a low serum T level and increasing the possibility of NGI causing biochemical phenocopy of HH?
APPROACH TO LOW-BORDERLINE SERUM T VALUE
INDICATIONS FOR TESTOSTERONE TREATMENT
AGING, OBESITY, AND OTHER COMORBIDITIES
Testosterone was marginally more effective than PDE5-inhibitors in respect of sexual functioning; albeit any superiority may not last beyond a year from initiation.
Testosterone did not improve vitality score, physical function, or cognitive function.
Testosterone increased coronary artery non-calcified plaque volume as assessed using computed tomographic angiography, indicating potential adverse CV risk.
Testosterone increased hemoglobin in both men with anemia of a known cause and in those having unexplained anemia.
Testosterone increased volumetric bone mineral density and the estimated bone strength of the spine and hip.
CAUSES OF HH THAT SHOULD NOT BE MISSED?
TESTOSTERONE TREATMENT AND ANDROGEN-SENSITIVE CANCERS
TESTOSTERONE AND CV DISEASE
MONITORING TESTOSTERONE THERAPY
DISCUSSION
Basic terminology: whether to remain with MH, or move to a new descriptor, TDS? We suggest sticking with MH.
Is MH (or TDS) a final diagnosis in its own right, or does it require a higher-order descriptor in order to be fully characterized and credible, such as HH due to opiate use, or PH due to Klinefelter syndrome? We suggest the latter.
HPG axis suppression due to co-morbidities such as obesity: should it be considered a form of HH like any other, and treated accordingly with testosterone; a form of functional, or non-organic HH, not usually treated with T, or is it instead better described as physiological NGI-effect for which a logical basis for testosterone prescribing, along with data on risks and benefits, are all notably lacking? We suggest the latter.
Case-finding paradigms: apart from men with core clinical features or risk factors (sexual dysfunction, infertility, gynecomastia, vasomotor symptoms, anemia, osteoporosis, absent or incomplete secondary sexual characteristic, characteristic syndromic features, or history of tumor, surgery, chemotherapy, or ionizing radiation affecting the HPT axis), who else—if anybody—should be screened for MH?
Specifically, should men with ongoing opiate use, prior non-prescription androgen use, or obesity/metabolic syndrome be screened for MH, even in the absence of relevant symptoms or signs, or is evidence of treatment-safety or -benefit inadequate for these? All but the AUS endorse testosterone prescribing for medically-justified opiate use [29,56].
Presence of obesity/metabolic syndrome: is this a valid justification for actively seeking-out MH, or instead, a reason to be very cautious in interpreting low serum T levels. We suggest the latter.
Diagnostic threshold: what should the serum T cut-off be for the diagnosis of MH? Among the clinical practice guidelines, the cut-off was variable and sometimes driven by studies looking mainly at certain symptoms national (Table 1) [27,57–60]. We suggest that individual laboratories engage with quality-control measures at a national level, aiming to achieve standardized reference ranges, rather than for guideline-writers to impose arbitrary thresholds.
Interpretation of a borderline T level: should age-adjusted ranges be adopted, or should the normal range derive from healthy younger men? We can see the merits of both approaches; the important thing is for the derivation of the reference range (healthy men or “all-comers”; age-adjusted or not?) to be clearly indicated on laboratory reports, as this will necessarily affect their interpretation by clinicians.
To what extent should levels of LH or SHBG influence the interpretation of serum total T; do free T calculations accurately correlate with values directly measured by equilibrium dialysis and does either measurement add extra diagnostic value? We believe that a consistently raised LH level unequivocally indicates Leydig cell insufficiency and, if associated with anemia, low bone density, painful gynecomastia, or treatment- refractory sexual dysfunction, merits consideration of testosterone treatment even when serum T is in the lower quartile, rather than being frankly below range. We also believe calculated free-T to be a useful adjunct to clinical decision-making when the SHBG level is an “outlier,” although we do not necessarily consider it to accurately reflect biochemical reality at tissue level.
Hct: what is the threshold value above which testosterone treatment is contraindicated and what is the maximum tolerated value on treatment and is venesection a safe and reasonable alternative to testosterone dose-reduction? We do not generally consider initiating testosterone treatment in men with Hct ≥48%, and would always titrate treatment so as to maintain it ≥50%, even if this means accepting a lower serum T value. This strategy has enabled us to avoid referring patients for venesection.
CV disease: do concerns only apply to men with frailty- or obesity-associated HH/NGI treated with testosterone, or also to men with organic, syndromic, or pathological MH? We strongly believe the former to be the case.
Prostate safety: is there any value in monitoring prostate safety for men having a verified diagnosis of MH and treated with testosterone, above any beyond the recommended level of screening in the background male population, and if so, does performing DRE offer any greater benefit than measurement of serum PSA level? Although firmly believing that greater interdisciplinary consensus is required in this area, we do not routinely perform DRE in our patients, but we do monitor PSA.
Old age: with increasing numbers of men surviving into their 9th and 10th decades, is there any chronological age above which concerns arise in respect of testosterone prescribing, even with a verified diagnosis of organic, syndromic, or pathological MH? In the absence of a ubiquitous male “andropause,” and with the overwhelming majority of older men broadly maintaining Leydig cell sensitivity to LH, we can envisage no upper age limit for prescribing testosterone when MH has been accurately diagnosed.
T2DM: given the strong association with PH, is there a causal relationship between T2DM and PH and what is the direction of causation? Unfortunately, research in the area of MH and T2DM has overwhelmingly focused on HH/NGI, rather than PH.
Table 1
| Guideline | T cutoff, nmol/L | Evidence for the cut off |
|---|---|---|
| ISSM, BSSM | <8 | Cross-sectional cohort study of 434 men (age 50–86 years) which showed that ED was more prevalent at this TT level [57] |
| AUA | <10.4 | Meta-analysis of RCTs including studies where TT <350 ng/dL, with a median baseline TT of 249 ng/dL and an interquartile range of 233–283 ng/dL [27,58] |
| EUA | <8 | A survey of 3,369 middle aged men (age 40–79 years) [59] |
| ES | <9.2 | Data from harmonized reference range from 100 healthy young nonobese men from 4 cohorts: the Framingham Heart Study, European Male Aging Study, Osteoporotic Fractures in Men Study, and Male Sibling Study of Osteoporosis [60] |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download