During the coronavirus disease 2019 (COVID-19) pandemic, the treatment of COVID-19 has evolved based on accumulated evidence from extensive research. Antibiotics such as macrolides and fluoroquinolones were investigated for their potential use for treatment of COVID-19 during the early stage of the pandemic,
12 but they displayed no clinical benefit.
34 Current guidelines do not recommend routinely prescribing antibiotics for COVID-19 patients.
56 Despite this, many medical institutions persisted in treating patients with antibiotics, and the treatment varied according to the judgment of individual medical staff. Furthermore, most of the antibiotics prescribed were administered without evidence of bacterial infection, and even some were broad spectrum antibiotics.
7 To evaluate whether empirical antibiotics therapy could improve clinical outcomes in COVID-19 patients requiring oxygen therapy, we conduct a retrospective propensity score-matching case-control study on patients who were transferred from a variety of hospitals with various initial treatments protocols.
Participants deemed eligible for this study consisted of moderate to severe, classified by National Institutes of Health criteria,
8 COVID-19 patients over 18 years old who were transferred to the Biocontainment Unit of Seoul National University Hospital between January 2020 and August 2021. Patients who were admitted to the intensive care unit (ICU) for mechanical ventilation or intubated within 24 hours of admission were excluded. When patients who were given empirical antibiotic therapy from other hospitals were admitted, medical staff evaluated the patients’ probability of bacterial infection and decided whether to continue or terminate antibiotic therapy. In addition, medical staff did not routinely initiate antibiotic therapy for patients admitted without evidence of bacterial infection. Thus, patients could be classified into two groups based on whether they were given empirical antibiotic therapy or not. Empirical antibiotic therapy is defined as the use of antibiotics within 48 hours after hospitalization prior to microbiological documentation of bacterial infection. Patients with bacterial or fungal detection in an isolate from a respiratory tract or blood culture sample, positive urinary antigen test for Streptococcus pneumoniae and legionella, or polymerase chain reaction detection of respiratory pathogens were all confirmed as having a bacterial infection.
We evaluated differences in the following clinical outcomes between groups with and without empirical antibiotic therapy: 1) number of days in the isolated ward, 2) total days with oxygen treatment, in addition to the proportion of patients, 3) with increased oxygen demand, 4) who required mechanical ventilation, and 5) who died during isolation. Patients who needed a high flow oxygen system or mechanical ventilation during treatment were defined as patients with increased oxygen demand. The variables considered in our analysis were: age, sex, coexisting conditions measured by the Charlson Comorbidity Index (CCI), immunosuppression status, and white blood cell (WBC) counts and C-reactive protein (CRP) concentrations taken within 48 hours of admission. Whether patients were administered remdesivir or corticosteroids was also taken into consideration. Immunocompromised status includes neutropenia, humoral immunodeficiency (hematological malignancy, post-splenectomy status and anti-CD20 antibody use) and cellular immunodeficiency (use of immunosuppressants).
Statistical comparisons between two groups were performed using two independent-sample t-tests, and a χ2 test. Count data were not normally distributed, so were subject to Loge transformation prior to analysis. Propensity score-matched analysis was performed to correct for the effect of clinical factors other than antibiotic therapy on prognosis. Comparisons after matching were performed using Wilcoxon signed-rank test and the McNemar test. A sensitivity analysis was subsequently conducted to validate the propensity score matching. Covariates were controlled by multivariate regression in all patients before matching and the results from conducting a generalized estimation equation (GEE) for each variable after matching. All statistical analyses were conducted using the R software package, version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria).
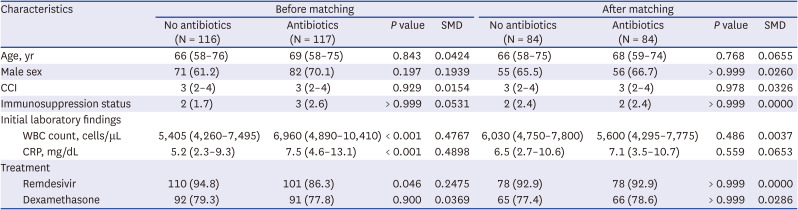
During the study period, 327 patients were referred to the study hospital for oxygen therapy. After excluding 94 patients because they were intubated within 24 hours, 233 patients were included in the analysis, with 116 patients in the group without empirical antibiotic therapy and 117 patients in the group receiving empirical antibiotic therapy. Patient characteristics are shown in
Table 1. WBC counts were higher in the group receiving empirical antibiotic therapy (6,960 vs. 5,405 cells/μL,
P < 0.001). CRP concentrations were also elevated in this group (7.5 vs. 5.2 mg/dL,
P < 0.001). In the group receiving empirical antibiotic therapy, the median duration of antibiotics treatment was 5.0 days (interquartile range [IQR], 2.5 to 9.0). Piperacillin/tazobactam was most frequently prescribed, followed by levofloxacin and moxifloxacin. Details of the empirical antibiotics prescribed are provided in
Supplementary Fig. 1.
Table 1
Baseline characteristics of the study population before and after propensity score matching
|
Characteristics |
Before matching |
After matching |
|
No antibiotics (N = 116) |
Antibiotics (N = 117) |
P value |
SMD |
No antibiotics (N = 84) |
Antibiotics (N = 84) |
P value |
SMD |
|
Age, yr |
66 (58–76) |
69 (58–75) |
0.843 |
0.0424 |
66 (58–75) |
68 (59–74) |
0.768 |
0.0655 |
|
Male sex |
71 (61.2) |
82 (70.1) |
0.197 |
0.1939 |
55 (65.5) |
56 (66.7) |
> 0.999 |
0.0260 |
|
CCI |
3 (2–4) |
3 (2–4) |
0.929 |
0.0154 |
3 (2–4) |
3 (2–4) |
0.978 |
0.0326 |
|
Immunosuppression status |
2 (1.7) |
3 (2.6) |
> 0.999 |
0.0531 |
2 (2.4) |
2 (2.4) |
> 0.999 |
0.0000 |
|
Initial laboratory findings |
|
|
|
|
|
|
|
|
|
WBC count, cells/μL |
5,405 (4,260–7,495) |
6,960 (4,890–10,410) |
< 0.001 |
0.4767 |
6,030 (4,750–7,800) |
5,600 (4,295–7,775) |
0.486 |
0.0037 |
|
CRP, mg/dL |
5.2 (2.3–9.3) |
7.5 (4.6–13.1) |
< 0.001 |
0.4898 |
6.5 (2.7–10.6) |
7.1 (3.5–10.7) |
0.559 |
0.0653 |
|
Treatment |
|
|
|
|
|
|
|
|
|
Remdesivir |
110 (94.8) |
101 (86.3) |
0.046 |
0.2475 |
78 (92.9) |
78 (92.9) |
> 0.999 |
0.0000 |
|
Dexamethasone |
92 (79.3) |
91 (77.8) |
0.900 |
0.0369 |
65 (77.4) |
66 (78.6) |
> 0.999 |
0.0286 |

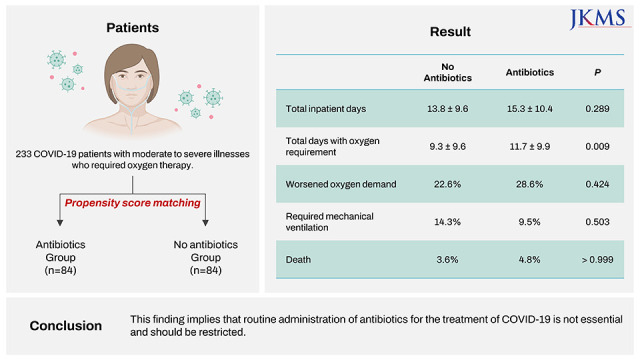
A total of 84 patients who received empirical antibiotic therapy were successfully matched to 84 patients in the group who did not receive empirical antibiotic therapy with the closest propensity scores. The baseline demographic and clinical characteristics were comparable between the two matched groups (
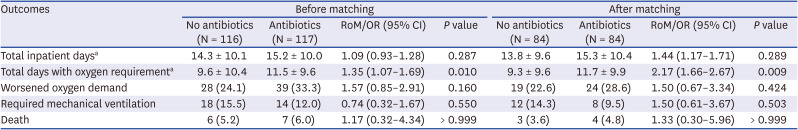
Table 1). There was no differences between the two groups in their length of stay (13.8 vs. 15.3 days,
P = 0.289), the proportion of patients with increased oxygen demand (22.6% [19/84] vs. 28.6% [24/84],
P = 0.424), the proportion of patients who required mechanical ventilation (14.3% [12/84] vs. 9.5% [8/84],
P = 0.503), and incidence of death during isolation (3.6% [3/84] vs. 4.8% [4/84],
P > 0.999). In addition, patients undergoing antibiotic therapy required more prolonged oxygen therapy than those not receiving antibiotic therapy (9.3 vs. 11.7 days,
P = 0.009;
Table 2). Sensitivity analysis supported these observations (
Supplementary Table 1).
Table 2
Comparison of outcomes between antibiotics use and non-use groups before and after propensity score matching
|
Outcomes |
Before matching |
After matching |
|
No antibiotics (N = 116) |
Antibiotics (N = 117) |
RoM/OR (95% CI) |
P value |
No antibiotics (N = 84) |
Antibiotics (N = 84) |
RoM/OR (95% CI) |
P value |
|
Total inpatient daysa
|
14.3 ± 10.1 |
15.2 ± 10.0 |
1.09 (0.93–1.28) |
0.287 |
13.8 ± 9.6 |
15.3 ± 10.4 |
1.44 (1.17–1.71) |
0.289 |
|
Total days with oxygen requirementa
|
9.6 ± 10.4 |
11.5 ± 9.6 |
1.35 (1.07–1.69) |
0.010 |
9.3 ± 9.6 |
11.7 ± 9.9 |
2.17 (1.66–2.67) |
0.009 |
|
Worsened oxygen demand |
28 (24.1) |
39 (33.3) |
1.57 (0.85–2.91) |
0.160 |
19 (22.6) |
24 (28.6) |
1.50 (0.67–3.34) |
0.424 |
|
Required mechanical ventilation |
18 (15.5) |
14 (12.0) |
0.74 (0.32–1.67) |
0.550 |
12 (14.3) |
8 (9.5) |
1.50 (0.61–3.67) |
0.503 |
|
Death |
6 (5.2) |
7 (6.0) |
1.17 (0.32–4.34) |
> 0.999 |
3 (3.6) |
4 (4.8) |
1.33 (0.30–5.96) |
> 0.999 |

In the group not receiving empirical antibiotic therapy, 28 patients began antibiotic treatment during hospitalization due to suspected bacterial infection. Of the 28 patients, bacterial infection was confirmed in eight (28.6%). The prevalence of documented bacterial infection in the group receiving empirical antibiotic therapy was 8.5% (10/117).
This study demonstrated that empirical antibiotic therapy does not improve clinical outcomes in COVID-19 patients. Antibiotic administration did not shorten the length of hospitalization or the length of time of oxygen therapy in moderate to severe COVID-19 patients. In addition, antibiotic therapy did not decrease the risk of increased oxygen demand, mechanical ventilation, or death. These results were robust after matching covariates that could affect the clinical outcome of COVID-19 patients. Consequently, antibiotic use itself did not improve the clinical course of COVID-19. As antibiotic use does not improve clinical outcomes and may even lead to adverse events, such as the development of
Clostridium difficile infection and the emergence of multi-drug resistant organisms,
9 proper antimicrobial stewardship practices are required.
The data reported in this manuscript are in accordance with the conclusions of previous studies. First, the incidence of combined bacterial infection in moderate to severe COVID-19 patients is low with only 7.7% (18/233) confirmed cases in moderate to severe COVID-19 patients. This is consistent with previous observations that less than 10% of COVID-19 patients presented with bacterial infection.
1011 Second, most COVID-19 patients received empirical antibiotic therapy without evidence of bacterial infection. Indeed, 46.4% (108/233) of patients with oxygen requirements were administered antibiotics improperly in our cohort. This is in agreement with previous studies demonstrating that unnecessary antibiotic treatment was frequently prescribed to COVID-19 patients.
712 Together, these results argue against prescription of empirical antibiotic therapy to COVID-19 patients since the overall proportion of bacterial infection is low. Therefore, empirical antibiotic therapy should be selectively considered if the clinician has a high suspicion of bacterial co-infection in a patient with radiological findings and/or inflammatory markers such as procalcitonin compatible with bacterial co-infection.
13
Fluoroquinolones and piperacillin/tazobactam were frequently prescribed empirically in COVID-19 patients, often without evidence of bacterial infection. Unnecessary use of broad-spectrum antibiotics leads to the emergence of multidrug-resistant organisms. Therefore, antibiotics should only be prescribed sparingly, based on the best available evidence for COVID-19 patients.
This study has a few limitations that require consideration when interpreting our data. Firstly, the study was retrospective. We minimized the shortcomings of a retrospective study design by conducting propensity score matching and sensitivity analysis. Propensity score matching was done with clinical factors known to be related to the progression of the disease and patients’ outcomes, but there are still other clinical factors such as Acute Physiology and Chronic Health Evaluation (APACHE) score or degrees of chest X-ray infiltration which may affect clinical outcomes. A second limitation was that the analysis was conducted on selected patients. Patients with severe illnesses who needed mechanical ventilation and mild patients who did not receive oxygen therapy were excluded. However, the need for antibiotic therapy in those patients was relatively clear, meaning we targeted the group whose need for antibiotic therapy has not been investigated.
In conclusion, this study showed that empirical antibiotic therapy does not improve clinical outcomes in moderate to severe COVID-19 patients. To the best of our knowledge, this study is the first to report the relationship between empirical antibiotic therapy and the prognosis of moderate to severe COVID-19 patients. These findings support current guidelines that routine administration of antibiotics for treatment of COVID-19 is not essential.
Ethics statement
The Institutional Review Board (IRB) at Seoul National University Hospital reviewed the study protocol and approved the study. The requirement for informed consent was waived due to the retrospective design of the study (IRB registration number H-2109-038-1252).