This article has been
cited by other articles in ScienceCentral.
Abstract
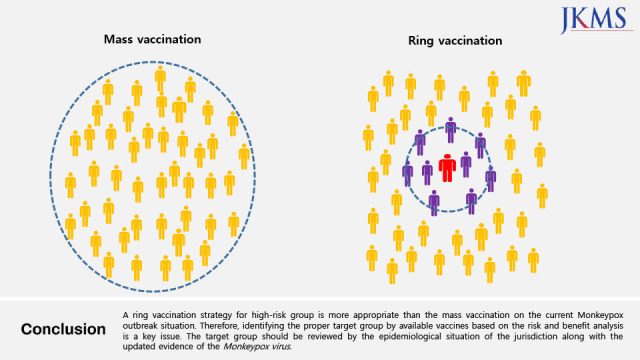
In June 2022, the first monkeypox case was reported as imported into Korea. The general public asked whether they should get vaccinated against monkeypox because of the recent COVID-19 vaccination experience. As of the current monkeypox outbreak situation, a ring vaccination strategy for the high-risk group is more appropriate than the mass population vaccination with smallpox vaccines. Therefore, identifying the proper target group by available vaccines based on the risk and benefit analysis is a key issue of the vaccination program. In addition, the target group should be reviewed by the epidemiological situation of the jurisdiction along with the updated evidence of the monkeypox virus on transmission dynamics, severity, and fatality.
Go to :

Graphical Abstract
Go to :

Keywords: Monkeypox, Vaccination, Smallpox Vaccine, Risk Assessment, Korea
Monkeypox outbreaks have been reported in non-endemic regions since May 2022 and the Republic of Korea was no exception. The first monkeypox case was confirmed in Korea on June 22, 2022.
1 Concerns around this newly emerging virus escalated because we are still experiencing the COVID-19 pandemic. Fortunately, vaccines are available against monkeypox, so the next question is what vaccination strategy will be applied. The World Health Organization does not recommend vaccination for the mass population based on current risk and benefit assessment.
2 Unlike the COVID-19 vaccination for the general population, the high-risk group of monkeypox virus exposure is the target and a “ring vaccination” strategy is recommended to control the outbreak, similarly to Ebola virus disease containment.
34 Smallpox vaccines such as ACAM2000 (live replicating second-generation smallpox vaccine), and JYNNEOS
TM (equivalent of IMVANEX
® in the European Union, non-replicating third-generation smallpox vaccine) are used against monkeypox because those vaccines are known to have 85% effectiveness by providing cross-immunity.
5
The high-risk group in terms of exposure is divided into pre- and post-exposure. The pre-exposure group is identified based on occupational probability; healthcare professionals, laboratory personnel and outbreak response team members are the target group of pre-exposure vaccination/prophylaxis (PrEP).
267 In addition to those occupational risk groups, the United Kingdom and Canada include gay, bisexual, and other men who have sex with men (GBMSM) as the PrEP target group based on their assessment of the outbreak situation.
89 Post-exposure prophylaxis (PEP) is recommended for contacts of confirmed cases. The exposure level from the cases should be assessed since a low level of exposure is not recommended for PEP.
26 This intervention needs to be administered ideally within four days of the last exposure until fourteen days to maximize the benefit.
267 Rigorous contact tracing capacity and a suppressed outbreak situation are prerequisites for the proper administration of PEP.
2
The Korea Disease Control and Prevention Agency (KDCA) has operated Vaccination Expert Advisory Group (VEAG) meetings for monkeypox vaccination since June 2, 2022. VEAG recommended PrEP and PEP for the high-risk target group. Thirty-five million doses of second-generation smallpox vaccine stockpiled by KDCA are being deployed for monkeypox vaccination. The vaccine was developed by a Korean pharmaceutical company; the HK inno.N’s Cell Culture Dry Smallpox Vaccine (CJ-50300) is derived from the vaccinia virus, the same strain used in ACAM2000.
10 The VEAG identified the high-risk target group based on the probability of exposure (
Table 1).
11 The GBMSM groups were not included as a high-risk group for PrEP by the VEAG based on the assessment of current outbreak situation of Korea. A risk-benefit assessment for using second-generation vaccines should be executed carefully with informed consent since the vaccine uses replicating live vaccinia virus. The VEAG strongly recommended rapidly importing the third-generation vaccine which has an improved side effect profile compared to the second-generation one.
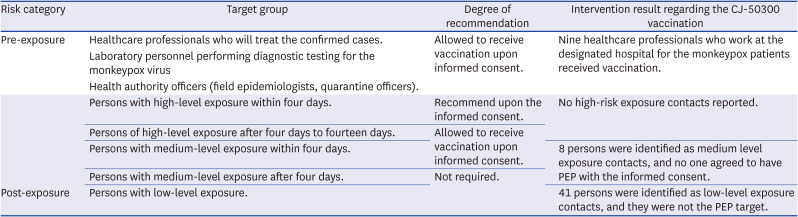
Table 1
Summary of recommendation and practice of monkeypox vaccination in Korea11
|
Risk category |
Target group |
Degree of recommendation |
Intervention result regarding the CJ-50300 vaccination |
|
Pre-exposure |
Healthcare professionals who will treat the confirmed cases. |
Allowed to receive vaccination upon informed consent. |
Nine healthcare professionals who work at the designated hospital for the monkeypox patients received vaccination. |
|
Laboratory personnel performing diagnostic testing for the monkeypox virus |
|
Health authority officers (field epidemiologists, quarantine officers). |
|
Post-exposure |
Persons with high-level exposure within four days. |
Recommend upon the informed consent. |
No high-risk exposure contacts reported. |
|
Persons of high-level exposure after four days to fourteen days. |
Allowed to receive vaccination upon informed consent. |
|
Persons with medium-level exposure within four days. |
8 persons were identified as medium level exposure contacts, and no one agreed to have PEP with the informed consent. |
|
Persons with medium-level exposure after four days. |
Not required. |
|
Persons with low-level exposure. |
41 persons were identified as low-level exposure contacts, and they were not the PEP target. |

After the first monkeypox case was confirmed in Korea on June 22, 2022, eight persons who had been seated close to the confirmed case in the airplane were identified as a medium-level exposure group according to the classification of exposure risk categories (
Table 2).
11 All eight contacts were informed about the PEP using second-generation vaccines along with the benefit and risk information. All of them agreed to not receive PEP before the fourth day from the last exposure.
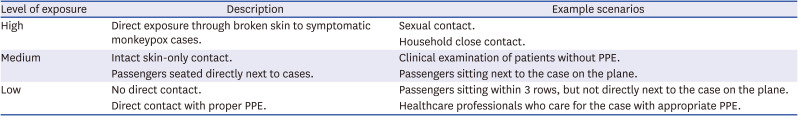
Table 2
Classification of exposure risk categories in Korea11
|
Level of exposure |
Description |
Example scenarios |
|
High |
Direct exposure through broken skin to symptomatic monkeypox cases. |
Sexual contact. |
|
Household close contact. |
|
Medium |
Intact skin-only contact. |
Clinical examination of patients without PPE. |
|
Passengers seated directly next to cases. |
Passengers sitting next to the case on the plane. |
|
Low |
No direct contact. |
Passengers sitting within 3 rows, but not directly next to the case on the plane. |
|
Direct contact with proper PPE. |
Healthcare professionals who care for the case with appropriate PPE. |

At the National Medical Center, nine healthcare professionals who work at the designated hospitals for the monkeypox patients received vaccination on June 27, 2022. All nine of them received the second-generation smallpox vaccine after giving their informed consent. An occlusive dressing was used for the vaccinated healthcare workers. All of them were instructed that they should maintain the vaccination site well covered while continued contact with patients is unavoidable.
10 The KDCA conducted active monitoring via phone or text messages for fourteen days about adverse reactions. All the data reported were the expected adverse reactions from CJ-50300’s clinical results.
12 Therefore, no significant adverse reactions have been reported.
The KDCA will procure 10,000 doses of JYNNEOSTM from the Danish Manufacturer, Bavarian Nordic. It is a sufficient amount for 5,000 people at 2 doses per one person. Since this third-generation vaccine has an improved side-effect profile, Korea will have more effective response measures to control the outbreak. When we decide on the vaccination target group in the near future, we should be reminded not only of the probability of exposure risk but also of the country’s specific epidemic situation. In the context of the limited outbreak situation in Korea, the PrEP and PEP target groups do not need to be broadened. Unlike in the United Kingdom and Canada, only one case has been confirmed and the prompt response to contain the outbreak has been working effectively. Therefore, special groups like GBMSM are not recommended for the PrEP. However, this decision will be reassessed based on the changing situation of outbreak in Korea.
Further studies are needed to determine appropriate vaccination target groups. In particular, more evidence for the mode of transmission, infectivity, morbidity, and mortality of the current monkeypox virus needs to be studied to optimize the disease containment strategy.