INTRODUCTION
Primary amenorrhea is defined as the absence of menarche until an age exceeding 2.5 standard deviations of the mean menarcheal age of the population. Recently, the average age at menarche in developed countries has stabilized at 12–13 years of age, however, the average age at thelarche showed a continuous downward trend.
12 Up to 98% of girls attaining menarche by 15 years, thus, the absence of menarche by this age, irrespective of secondary sex characteristics, is statistically uncommon and requires thorough investigation.
3 The absence of breast development until 13 years of age also requires assessment to rule out significant gonadal or hypothalamo-pituitary failure, which may impair adolescent bone health and future fertility.
456 Considering normal hypothalamo-pituitary-ovarian axis and normal genital anatomy are necessary for normal menstruation, etiologies of amenorrhea are commonly classified into hypergonadotropic hypogonadism, hypogonadotropic hypogonadism, and eugonadism, mainly based on hormone test and imaging. Abnormal development of sex is another category.
Scientific data on the prevalence of specific etiologies causing primary amenorrhea remain insufficient and inconclusive. Reindollar et al.
4 analyzed delayed sexual development including primary amenorrhea in the U.S. population and demonstrated that gonadal dysgenesis was the most frequent cause of primary amenorrhea. In contrast, studies conducted in Asian countries reported that Müllerian agenesis is the leading cause of primary amenorrhea.
78910 Although the etiologic causes differ according to the population, the difference may be due to the relatively small sample sizes analyzed. In addition, in single-center studies, the distributions of diagnoses can be biased according to specialized medical field and the geographic location of the medical facility.
Among the etiologies of primary amenorrhea, polycystic ovarian syndrome (PCOS) and functional hypothalamic amenorrhea are considered to be influenced by environmental factors such as obesity and nutritional status.
1112 Although over-nutrition is a non-pathognomonic aspect of PCOS, it plays roles in PCOS pathogenesis via insulin resistance and worsening hyperandrogenism.
13 Recently, the incidence of eating disorders is increasing worldwide and the cost of medical expenditures due to eating disorders in South Korea increased between 2010 and 2015.
14 In addition, the prevalence of childhood obesity in Korea increased significantly from 8.7% in 2007 to 15.0% in 2017.
15 Although changes in obesity and nutritional status presumably increase the incidence of PCOS and functional hypothalamic amenorrhea, there have been no studies regarding the secular changes of these problems as a cause of primary amenorrhea.
In the present study we present an accurate characterization of the etiologic causes of primary amenorrhea in the Korean population and elucidate secular changes in the occurrences of specific disorders causing primary amenorrhea between 2000 and 2016.
Go to :

METHODS
Study design and subjects
This retrospective multicenter study included eight university-affiliated hospitals located in Seoul, South Korea. To select eligible subjects, the investigators searched for the diagnostic codes for primary amenorrhea as well as representative diagnoses that cause primary amenorrhea between January 2000 and December 2016, using keywords in the hospital databases of the participating institutions. Primary amenorrhea was principally defined as an absence of menarche by 15 years of age irrespective of secondary sex characteristics or an absence of menarche by 13 years of age in patients without developing secondary sex characteristics. Since primary amenorrhea is mostly diagnosed in the teens to early 20s in the Korean population,
8 subjects diagnosed with primary amenorrhea at ages older than 30 years were excluded from this study because of concerns that their inclusion would reduce the accuracy of the diagnosis. In order to accurately define the secular trend of etiologic causes of primary amenorrhea, we excluded subjects diagnosed with definite causes of primary amenorrhea such as Turner syndrome or outflow tract obstruction if they did not reach age 13 (without breast development) or age 15 (with breast development) within the study period.
The inclusion criteria were: 1) subjects diagnosed with primary amenorrhea between January 2000 and December 2016, and 2) subjects who were surgically treated for outflow tract obstruction before 15 years of age if they reached the age of 15 years within the study period. The exclusion criteria were: 1) subjects > 30 years at the time of diagnosis, 2) subjects diagnosed with definite causes of primary amenorrhea, such as Turner syndrome or outflow tract obstruction, if they did not reach age 13 (without breast development) or age 15 (with breast development) within the study period, and 3) subjects with poor diagnosis accuracy or lack of evidence in medical records.
Data collection
Data were obtained from the medical records for patients who were diagnosed with primary amenorrhea between January 2000 and December 2016. Age, height, weight, body mass index (BMI), presence of spontaneous breast and pubic hair development, and clinical diagnosis were recorded for all enrolled patients. Basal serum levels of gonadotropins, sex hormones, thyroid profiles, and prolactin were recorded, and cytogenetic data and outcomes of imaging studies were collected, when available. All data were recorded using specifically designed case report forms (CRFs), which were developed by the investigators and uploaded to the Bethesda Soft Inc. website (Seoul, Korea;
www.bethesdasoft.co.kr). Data were collected at each institution by specialists in gynecologic endocrinology, based on the shared criteria. In order to exclude the possibility that a single patient visited more than one institution, subjects with identical initials, date of birth, and diagnosis were considered as one patient.
Characterization of etiologic diagnoses causing primary amenorrhea
The etiologic causes or diagnoses of amenorrhea were verified by experienced gynecologic endocrinologists who were affiliated with each participating institution. These experts conducted verification by reviewing the detailed medical history, physical examination findings, hormonal and cytogenetic results, imaging studies and histopathologic data for the patients. After data from all institutions has been collected, designated investigators conducted secondary verification by reviewing data, and if the results clearly did not match the diagnosis, it was inquired at each institution for reconfirmation and correction. Considering descriptions by the World Health Organization, etiologies of primary amenorrhea were classified into four categories mainly based on estrogen and gonadotropin: Hypogonadotropic hypogonadism (group 1) means decreased estrogen production with low follicle-stimulating hormone (FSH). Eugonadism (group 2) means the presence of estrogen production with normal FSH levels. Hypergonadotropic hypogonadism (group 3) means elevated FSH level. Abnormal development of sex, excluding pure gonadal dysgenesis of the XX or XY karyotype and Turner syndrome, was another category (group 4).
Statistical analysis
The median values of body weight, height, BMI, at the time of diagnosis were compared between categories or specific diseases in subjects diagnosed with primary amenorrhea. In addition, we assessed secular trends of the etiology of primary amenorrhea as well as developmental status by sub-grouping the subjects into three periods based on the year of diagnosis (Period 1: 2000–2006; Period 2: 2007–2011; and Period 3: 2012–2016).
Descriptive statistics were provided to summarize demographic data including age, height, weight, BMI. Continuous variables were presented as median (interquartile range [IQR]) and analyzed using the Kruskal-Wallis test after confirming normality, and linear regression analyses were used to evaluate differences according to the etiology of primary amenorrhea. Bonferroni’s correction was applied to significances obtained from regression analysis between subgroups. Categorical variables were presented as frequency and proportion (%) and differences were tested using χ2 tests with or without missing categories. The proportions of the etiologies of primary amenorrhea were assessed by year of diagnosis using tests for trend. P values < 0.05 divided by the number of pairwise comparisons were considered statistically significant. Statistical analyses were performed using Stata 16.0 (StataCorp LLC, College Station, TX, USA).
Ethics statement
The study protocol was approved by the Institutional Review Boards of the participating institutions before the review of patient materials (IRB No. 2018-03-131). The requirement for informed consent was waived due to the retrospective nature of the study. This study was conducted in accordance with the principles of the Declaration of Helsinki.
Go to :

RESULTS
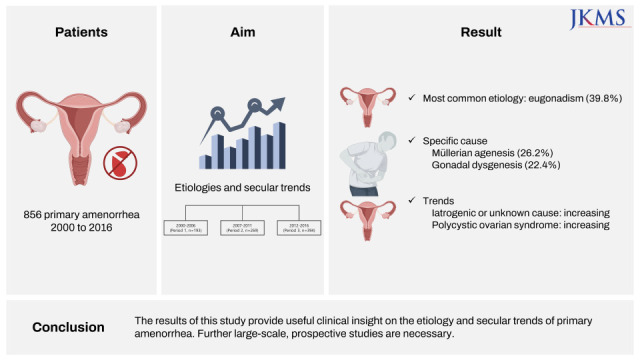
Among 1,060 initially enrolled subjects, etiologic diagnoses were successfully confirmed and categorized in 856 cases, while 204 cases were finally excluded. Reasons for exclusion were inadequate age criteria (n = 139), inadequate timing at diagnosis (n = 49), and poor diagnosis accuracy or lack of evidence (n = 16) (
Fig. 1).
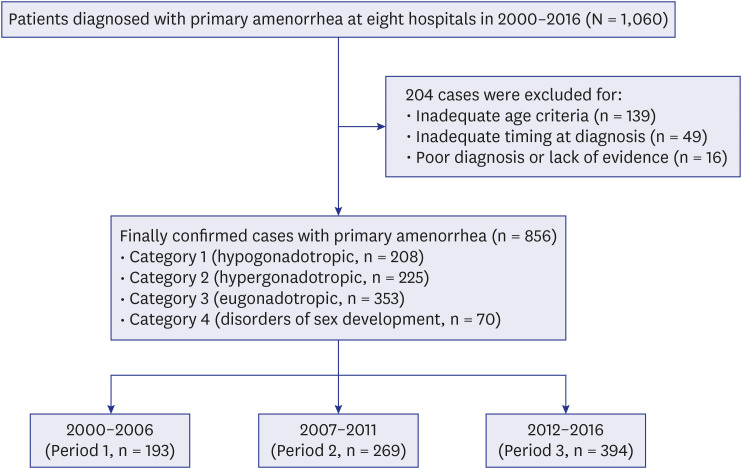 | Fig. 1 Study population.
|
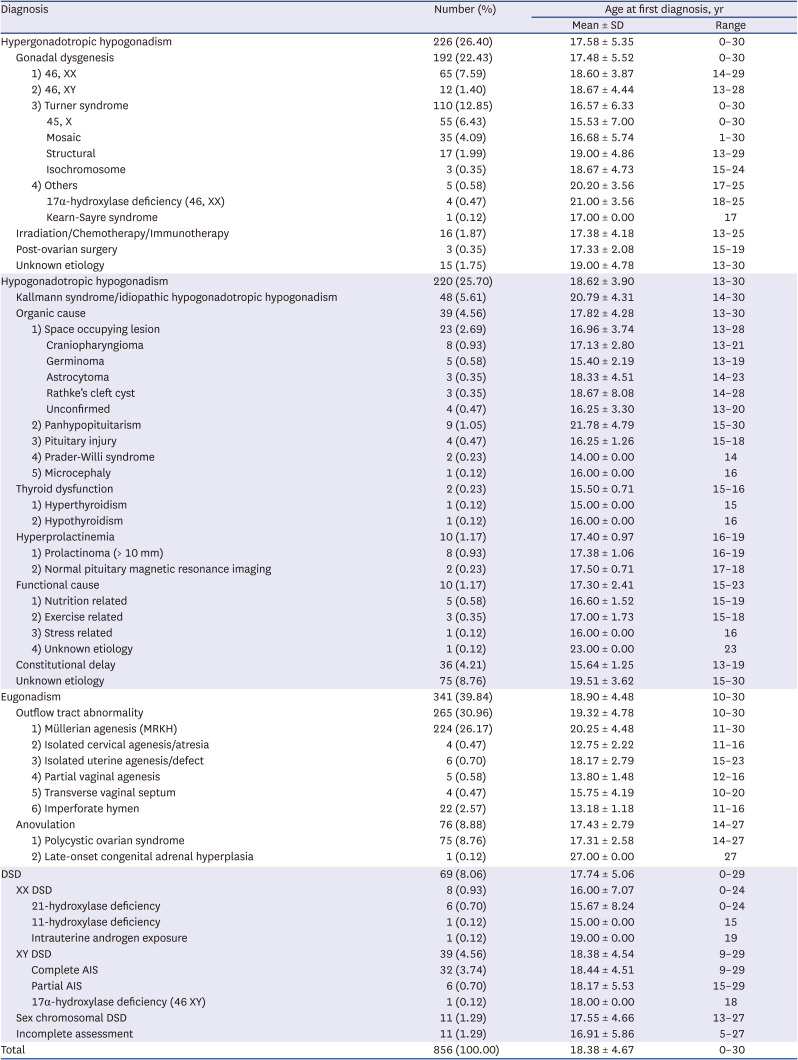
Table 1 demonstrates the etiologies of primary amenorrhea in 856 patients. Eugonadism was the most common category (39.8%, 341/856), whereas disorders of sex development were present in less than 10% of the patients (8.1%, 69/856). Hypergonadotropic and hypogonadotropic hypogonadism accounted for about half of the diagnoses. Müllerian agenesis was the most common specific cause (26.2%), followed by gonadal dysgenesis (22.4%).
Table 1
Diagnostic distribution of primary amenorrhea, 2000–2016
|
Diagnosis |
Number (%) |
Age at first diagnosis, yr |
|
Mean ± SD |
Range |
|
Hypergonadotropic hypogonadism |
226 (26.40) |
17.58 ± 5.35 |
0–30 |
|
Gonadal dysgenesis |
192 (22.43) |
17.48 ± 5.52 |
0–30 |
|
|
1) 46, XX |
65 (7.59) |
18.60 ± 3.87 |
14–29 |
|
|
2) 46, XY |
12 (1.40) |
18.67 ± 4.44 |
13–28 |
|
|
3) Turner syndrome |
110 (12.85) |
16.57 ± 6.33 |
0–30 |
|
|
|
45, X |
55 (6.43) |
15.53 ± 7.00 |
0–30 |
|
|
|
Mosaic |
35 (4.09) |
16.68 ± 5.74 |
1–30 |
|
|
|
Structural |
17 (1.99) |
19.00 ± 4.86 |
13–29 |
|
|
|
Isochromosome |
3 (0.35) |
18.67 ± 4.73 |
15–24 |
|
|
4) Others |
5 (0.58) |
20.20 ± 3.56 |
17–25 |
|
|
|
17α-hydroxylase deficiency (46, XX) |
4 (0.47) |
21.00 ± 3.56 |
18–25 |
|
|
|
Kearn-Sayre syndrome |
1 (0.12) |
17.00 ± 0.00 |
17 |
|
Irradiation/Chemotherapy/Immunotherapy |
16 (1.87) |
17.38 ± 4.18 |
13–25 |
|
Post-ovarian surgery |
3 (0.35) |
17.33 ± 2.08 |
15–19 |
|
Unknown etiology |
15 (1.75) |
19.00 ± 4.78 |
13–30 |
|
Hypogonadotropic hypogonadism |
220 (25.70) |
18.62 ± 3.90 |
13–30 |
|
Kallmann syndrome/idiopathic hypogonadotropic hypogonadism |
48 (5.61) |
20.79 ± 4.31 |
14–30 |
|
Organic cause |
39 (4.56) |
17.82 ± 4.28 |
13–30 |
|
|
1) Space occupying lesion |
23 (2.69) |
16.96 ± 3.74 |
13–28 |
|
|
|
Craniopharyngioma |
8 (0.93) |
17.13 ± 2.80 |
13–21 |
|
|
|
Germinoma |
5 (0.58) |
15.40 ± 2.19 |
13–19 |
|
|
|
Astrocytoma |
3 (0.35) |
18.33 ± 4.51 |
14–23 |
|
|
|
Rathke’s cleft cyst |
3 (0.35) |
18.67 ± 8.08 |
14–28 |
|
|
|
Unconfirmed |
4 (0.47) |
16.25 ± 3.30 |
13–20 |
|
|
2) Panhypopituitarism |
9 (1.05) |
21.78 ± 4.79 |
15–30 |
|
|
3) Pituitary injury |
4 (0.47) |
16.25 ± 1.26 |
15–18 |
|
|
4) Prader-Willi syndrome |
2 (0.23) |
14.00 ± 0.00 |
14 |
|
|
5) Microcephaly |
1 (0.12) |
16.00 ± 0.00 |
16 |
|
Thyroid dysfunction |
2 (0.23) |
15.50 ± 0.71 |
15–16 |
|
|
1) Hyperthyroidism |
1 (0.12) |
15.00 ± 0.00 |
15 |
|
|
2) Hypothyroidism |
1 (0.12) |
16.00 ± 0.00 |
16 |
|
Hyperprolactinemia |
10 (1.17) |
17.40 ± 0.97 |
16–19 |
|
|
1) Prolactinoma (> 10 mm) |
8 (0.93) |
17.38 ± 1.06 |
16–19 |
|
|
2) Normal pituitary magnetic resonance imaging |
2 (0.23) |
17.50 ± 0.71 |
17–18 |
|
Functional cause |
10 (1.17) |
17.30 ± 2.41 |
15–23 |
|
|
1) Nutrition related |
5 (0.58) |
16.60 ± 1.52 |
15–19 |
|
|
2) Exercise related |
3 (0.35) |
17.00 ± 1.73 |
15–18 |
|
|
3) Stress related |
1 (0.12) |
16.00 ± 0.00 |
16 |
|
|
4) Unknown etiology |
1 (0.12) |
23.00 ± 0.00 |
23 |
|
Constitutional delay |
36 (4.21) |
15.64 ± 1.25 |
13–19 |
|
Unknown etiology |
75 (8.76) |
19.51 ± 3.62 |
15–30 |
|
Eugonadism |
341 (39.84) |
18.90 ± 4.48 |
10–30 |
|
Outflow tract abnormality |
265 (30.96) |
19.32 ± 4.78 |
10–30 |
|
|
1) Müllerian agenesis (MRKH) |
224 (26.17) |
20.25 ± 4.48 |
11–30 |
|
|
2) Isolated cervical agenesis/atresia |
4 (0.47) |
12.75 ± 2.22 |
11–16 |
|
|
3) Isolated uterine agenesis/defect |
6 (0.70) |
18.17 ± 2.79 |
15–23 |
|
|
4) Partial vaginal agenesis |
5 (0.58) |
13.80 ± 1.48 |
12–16 |
|
|
5) Transverse vaginal septum |
4 (0.47) |
15.75 ± 4.19 |
10–20 |
|
|
6) Imperforate hymen |
22 (2.57) |
13.18 ± 1.18 |
11–16 |
|
Anovulation |
76 (8.88) |
17.43 ± 2.79 |
14–27 |
|
|
1) Polycystic ovarian syndrome |
75 (8.76) |
17.31 ± 2.58 |
14–27 |
|
|
2) Late-onset congenital adrenal hyperplasia |
1 (0.12) |
27.00 ± 0.00 |
27 |
|
DSD |
69 (8.06) |
17.74 ± 5.06 |
0–29 |
|
XX DSD |
8 (0.93) |
16.00 ± 7.07 |
0–24 |
|
|
21-hydroxylase deficiency |
6 (0.70) |
15.67 ± 8.24 |
0–24 |
|
|
11-hydroxylase deficiency |
1 (0.12) |
15.00 ± 0.00 |
15 |
|
|
Intrauterine androgen exposure |
1 (0.12) |
19.00 ± 0.00 |
19 |
|
XY DSD |
39 (4.56) |
18.38 ± 4.54 |
9–29 |
|
|
Complete AIS |
32 (3.74) |
18.44 ± 4.51 |
9–29 |
|
|
Partial AIS |
6 (0.70) |
18.17 ± 5.53 |
15–29 |
|
|
17α-hydroxylase deficiency (46 XY) |
1 (0.12) |
18.00 ± 0.00 |
18 |
|
Sex chromosomal DSD |
11 (1.29) |
17.55 ± 4.66 |
13–27 |
|
Incomplete assessment |
11 (1.29) |
16.91 ± 5.86 |
5–27 |
|
Total |
856 (100.00) |
18.38 ± 4.67 |
0–30 |

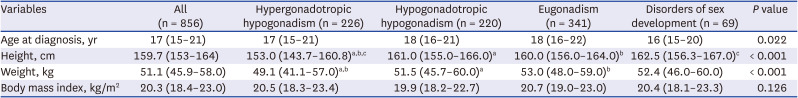
Table 2 compares the clinical characteristics of the study subjects according to the etiologies of primary amenorrhea. Median age at first diagnosis was 17 years (IQR, 15–21 years). Although women with hypergonadotropic hypogonadism were more likely to have lower height and weight compared to those with other diagnoses (
P < 0.001), BMI was comparable among all four groups.
Table 2
Baseline characteristics according to the diagnosis of primary amenorrhea
|
Variables |
All (n = 856) |
Hypergonadotropic hypogonadism (n = 226) |
Hypogonadotropic hypogonadism (n = 220) |
Eugonadism (n = 341) |
Disorders of sex development (n = 69) |
P value |
|
Age at diagnosis, yr |
17 (15–21) |
17 (15–21) |
18 (16–21) |
18 (16–22) |
16 (15–20) |
0.022 |
|
Height, cm |
159.7 (153–164) |
153.0 (143.7–160.8)a,b,c
|
161.0 (155.0–166.0)a
|
160.0 (156.0–164.0)b
|
162.5 (156.3–167.0)c
|
< 0.001 |
|
Weight, kg |
51.1 (45.9–58.0) |
49.1 (41.1–57.0)a,b
|
51.5 (45.7–60.0)a
|
53.0 (48.0–59.0)b
|
52.4 (46.0–60.0) |
< 0.001 |
|
Body mass index, kg/m2
|
20.3 (18.4–23.0) |
20.5 (18.3–23.4) |
19.9 (18.2–22.7) |
20.7 (19.0–23.0) |
20.4 (18.1–23.3) |
0.126 |

Table 3 presents secular trends of the etiologies of primary amenorrhea. With respect to hypergonadotropic hypogonadism, proportions of irradiation/chemotherapy and unknown etiology increased in 2012–2016, showing significantly different distributions (
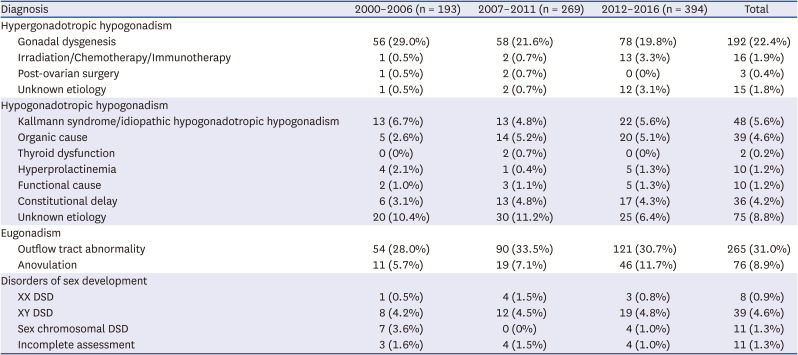
P < 0.001). It appeared that the proportion of anovulation increased with time, but the change did not reach statistical significance. No significant findings were observed in the distributions of other diagnoses of primary amenorrhea.
Table 3
Secular trends in primary amenorrhea by diagnosis
|
Diagnosis |
2000–2006 (n = 193) |
2007–2011 (n = 269) |
2012–2016 (n = 394) |
Total |
|
Hypergonadotropic hypogonadism |
|
|
|
|
|
Gonadal dysgenesis |
56 (29.0%) |
58 (21.6%) |
78 (19.8%) |
192 (22.4%) |
|
Irradiation/Chemotherapy/Immunotherapy |
1 (0.5%) |
2 (0.7%) |
13 (3.3%) |
16 (1.9%) |
|
Post-ovarian surgery |
1 (0.5%) |
2 (0.7%) |
0 (0%) |
3 (0.4%) |
|
Unknown etiology |
1 (0.5%) |
2 (0.7%) |
12 (3.1%) |
15 (1.8%) |
|
Hypogonadotropic hypogonadism |
|
|
|
|
|
Kallmann syndrome/idiopathic hypogonadotropic hypogonadism |
13 (6.7%) |
13 (4.8%) |
22 (5.6%) |
48 (5.6%) |
|
Organic cause |
5 (2.6%) |
14 (5.2%) |
20 (5.1%) |
39 (4.6%) |
|
Thyroid dysfunction |
0 (0%) |
2 (0.7%) |
0 (0%) |
2 (0.2%) |
|
Hyperprolactinemia |
4 (2.1%) |
1 (0.4%) |
5 (1.3%) |
10 (1.2%) |
|
Functional cause |
2 (1.0%) |
3 (1.1%) |
5 (1.3%) |
10 (1.2%) |
|
Constitutional delay |
6 (3.1%) |
13 (4.8%) |
17 (4.3%) |
36 (4.2%) |
|
Unknown etiology |
20 (10.4%) |
30 (11.2%) |
25 (6.4%) |
75 (8.8%) |
|
Eugonadism |
|
|
|
|
|
Outflow tract abnormality |
54 (28.0%) |
90 (33.5%) |
121 (30.7%) |
265 (31.0%) |
|
Anovulation |
11 (5.7%) |
19 (7.1%) |
46 (11.7%) |
76 (8.9%) |
|
Disorders of sex development |
|
|
|
|
|
XX DSD |
1 (0.5%) |
4 (1.5%) |
3 (0.8%) |
8 (0.9%) |
|
XY DSD |
8 (4.2%) |
12 (4.5%) |
19 (4.8%) |
39 (4.6%) |
|
Sex chromosomal DSD |
7 (3.6%) |
0 (0%) |
4 (1.0%) |
11 (1.3%) |
|
Incomplete assessment |
3 (1.6%) |
4 (1.5%) |
4 (1.0%) |
11 (1.3%) |

Go to :

DISCUSSION
The present multicenter study describes the etiologies of primary amenorrhea during long-term period in South Korea. Although primary amenorrhea is uncommon, considering the negative impacts on affected patients in both physical and psychological aspects, much more attention should be paid to this problem. As a first step, it is important to address etiologies specific to each region or country.
In the present study, the most common specific cause of primary amenorrhea was Müllerian agenesis (26.2%), followed by gonadal dysgenesis (22.4%). This finding is consistent with those of previous studies reporting that Müllerian agenesis and gonadal dysgenesis are common causes of primary amenorrhea, but the frequency of each etiology differs across studies. Among 295 women in Thailand, the most common causes of primary amenorrhea were Müllerian agenesis (39.7%), gonadal dysgenesis (35.3%), and hypogonadotropic hypogonadism (9.2%).
7 Among 105 women in India, the most common causes of primary amenorrhea were Müllerian anomalies (47%) and gonadal dysgenesis (20.5%).
9 In Pakistan, Müllerian agenesis was also the most common cause (46%) among 100 patients with primary amenorrhea.
16 In contrast, in a sample of 252 U.S. patients, the most common causes of primary amenorrhea were gonadal dysgenesis (50%), hypothalamic or pituitary problems (25%), and anatomic defects (20%).
4 According to the Practice Committee of the American Society for Reproductive Medicine, hypergonadotropic hypogonadism was the most common cause (40%) of primary amenorrhea, and the frequencies of hypothalamic or pituitary problems and anatomic defects were similar (30%).
17
Based on the literature, the leading cause of primary amenorrhea may differ between Asian and western countries. The exact reason for this difference is unclear, but both genetic and environmental factors may play a role. Most important reason may be genetic, and even anatomical cause, such as Müllerian anomalies, also can be affected by the genetic factor. However, based on a recent survey in Japan, the most common cause of primary amenorrhea was abnormal karyotype (26.4%), followed by Müllerian agenesis (15.8%).
18 In addition, even in a previous study of 132 Korean women with primary amenorrhea, the most frequent cause was gonadal dysgenesis (28%), followed by Mayer-Rokitansky-Küster-Hauser syndrome (20%).
8 Therefore, in addition to genetic and environmental factors, heterogeneity across studies could result in difference. Also, there could be some challenges for the proper diagnosis of etiologies according to resources or facilities in each countries, especially regarding uncommon causes. These findings suggest that necessity of large-scale prospective studies regarding etiology of primary amenorrhea distinguished by population or region.
The functional cause of hypogonadotropic hypogonadism, mainly nutrition-related, was less common in the present study (1.2%) compared to previous studies in the U.S. (3%), Japan (8%), and India (14.7%).
91718 It is probable that some patients who we classified as “unknown etiology” may actually have “functional cause” amenorrhea, resulting in the relatively low frequency of the latter in our study. However, the frequency of functional hypothalamic amenorrhea has been reported to be very low in other studies of primary amenorrhea, and it is difficult to ascertain whether there is a clear difference among studies. Constitutional delay is a self-limited form of delayed puberty that can be diagnosed only after achieving normal puberty and reproductive function. Constitutional delay was commonly described as the most common cause, accounting for 30%, of delayed puberty in females.
19 On the other side, in the study of Timmreck and Reindollar
5 analyzing 266 patients with primary amenorrhea, the proportion of constitutional delay was only 6%, similar to the results of our study (4.2%). The reason for the difference in prevalence of constitutional delay among studies is unclear; however, the participation of only university affiliated hospitals in this study could be a factor that made the prevalence of self-limited constitutional delay lower.
Interestingly, our analysis of secular trends demonstrated that the frequency of anovulation, mostly due to PCOS, appeared to increase from 5.7% in Period I (2000–2006) to 11.7% in Period 3 (2012–2016), although the difference did not reach statistical significance. PCOS is a relatively uncommon cause of primary amenorrhea, and its frequency ranges from 1.4% to 6.8% in patients with primary amenorrhea.
57891020 Although there is a slight discrepancy in data source, the prevalence of pediatric obesity in Korea has also been increasing over previous decades. In a study using Korean National School Health Examination data, the prevalence of obesity among girls aged 6–18 years increased from 6.6% in 2007 to 12.0% in 2017.
15 Considering the relationship between childhood obesity and the development of adolescent PCOS,
212223 the trend of increasing obesity among Korean adolescents may have contributed to the increased incidence of adolescent PCOS over time. However, in order to determine whether the frequency of PCOS is related to the increase in primary amenorrhea, a prospective study with subjects diagnosed using unified PCOS diagnostic criteria should be conducted.
This study has several strengths. First, it is the largest study evaluating the etiologies of primary amenorrhea. Most previous studies analyzed less than 300 patients. Second, it is a multicenter study that could be representative of Korean patients as a whole. In Korea, almost all patients with primary amenorrhea are referred to a tertiary-care university hospital. Eight university hospitals in the current study are large-scale referral centers in Korea, and may therefore represent nationwide information regarding primary amenorrhea, compared with studies from a single institution. In addition, we addressed secular trends of causes of amenorrhea in this study for the first time. Until now, all studies were cross-sectional. Considering that environmental factors or even genetic factors can change with time, our study provides useful clinical information.
This study also has some limitations. It is a retrospective study, and data were obtained from medical records. Therefore, missing data were unavoidable. It might affect some results of the present study. In addition, not all patients who were diagnosed with primary amenorrhea during the study period were included into the study. Moreover, grouping the subjects into three periods is arbitrary and a number of the subjects is relatively small for most etiologies in each periods, which might not be appropriate for assessing secular trends of the etiologies.
In conclusion, the results of this study provide useful clinical insights into the etiology of primary amenorrhea. Further large-scale, prospective, long-term studies are warranted to gain a better understanding of the etiologies of primary amenorrhea.
Go to :








 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download