This article has been
cited by other articles in ScienceCentral.
Abstract
Background
Glucocorticoids are one of the current standard agents for moderate to severe coronavirus disease 2019 (COVID-19) treatment based on the RECOVERY trial. Data on the real clinical application of steroids for COVID-19 are scarce and will help guide the optimal use of steroids. We described the current prescription pattern of steroids for COVID-19 and investigated the factors related to specific practices.
Methods
All adults aged ≥ 19 years who were diagnosed with COVID-19 by real-time reverse transcription-polymerase chain reaction and admitted to one of 3 study hospitals from 8 December 2020 to 30 June 2021 were enrolled. Demographic and clinical data, including medications and oxygen therapy, were retrospectively collected from electronic medical records. The severity of comorbidities and COVID-19 were measured. The subjects were divided into steroid and nonsteroid groups, and the steroid group was then subdivided into standard and higher/longer groups.
Results
Among a total of 805 patients, 217 (27.0%) were treated with steroids. The steroid group showed a higher rate of oxygen therapy (81.1% vs. 2.7%), more concomitant use of remdesivir (77.4% vs. 1.4%) or antibiotics (79.3% vs. 4.3%), and a higher proportion of high risk according to National Early Warning Score-2 score (30.0% vs. 0.9%) or severe risk according to National Institute of Allergy and Infectious Disease Ordinal Scale score (81.1% vs. 2.7%) than the nonsteroid group. The mortality of the steroid group was 4.6%. In the steroid group, 82.5% received a standard or lower dose of steroids within ten days, and 17.5% (38/217) received a higher or longer dose of steroids. Multivariate analysis showed that initial lymphopenia (adjusted odds ratio [aOR], 0.94; 95% confidence interval [CI], 0.89–0.99) and high level of lactate dehydrogenase (LDH) (aOR, 1.00; 95% CI, 1.00–1.01) were independent risk factors for higher doses or longer steroid use.
Conclusion
The dose and duration of steroids were in line with current guidelines in 82.5% of COVID-19 patients, but the outliers may need tailored therapy according to surrogate markers, such as initial lymphopenia or high level of LDH.
Keywords: COVID-19, SARS-CoV-2, Steroid
INTRODUCTION
In the pandemic of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2),
12 treatment strategies for the management of moderate to severe patients have been available. Remdesivir, a novel nucleotide analog, is now recommended for 5 days and may be extended to 10 days in certain situations for COVID-19 patients with pneumonia and the need for oxygen therapy.
34567 Other agents, including Janus kinase inhibitors or monoclonal antibodies, are also recommended as treatment options.
5678 The improvement in 28-day mortality with corticosteroids, including dexamethasone, in COVID-19 patients with oxygen requirements was evidenced by the RECOVERY trial,
9 and dexamethasone 6 mg or an equivalent dose of alternative glucocorticoids is now recommended for 10 days or until discharge for moderate to severe COVID-19 patients.
567 The oral antiviral agents nirmatrelvir/ritonavir and molnupiravir have recently been added to the available list. With a handful of anti-SARS-CoV-2 drugs, we need the best treatment strategies to guide the use of available drugs.
Corticosteroids are cheap and readily available for use. Lower or higher dosing, longer use and restart after initial stop are not infrequent in clinical practice in response to subtle changes in the individual clinical severity of COVID-19 patients within predefined severity stages, severe or critical disease. The aim of this study was to describe the current prescription pattern of steroids for COVID-19 patients in real clinical practice and investigate the outcome and related factors of such flexible steroid use outside clinical trial settings.
METHODS
Study design and data collection
All adult patients aged ≥ 19 years who were diagnosed with COVID-19 by real-time reverse transcription-polymerase chain reaction (RT-PCR) with symptoms or without symptoms and admitted or transferred from other hospitals or residential treatment centers (RTCs) to one of three study hospitals, Boramae Medical Center (BMC), Veterans Health Service Medical Center (VHSMC) and Korea Cancer Center Hospital (KCCH), were enrolled. The Korean government revised the policy on 7 December 2020 to isolate the patients for 10 days. Therefore, only patients who were admitted or transferred from 8 December 2020 were enrolled. Patients who received immunosuppressants other than steroids or participated in the clinical trials related to COVID-19 were excluded. Because of the admission capacity of each study hospital, the durations of data collection were different: from 7 December 2020 to 28 February 2021 at BMC, from 1 March 2021 to 30 April 2021 at VHSMC and from 23 December 2020 to 30 June 2021 at KCCH.
Clinical data were retrospectively collected using electronic medical records, and the variables included demographics, route of admission (community, transfer from other hospitals or RTC), comorbidities and weighted Charlson Comorbidity Index (CCI) score,
10 types of symptoms and their onset time, oxygen saturation within 24 hours of admission, types of oxygen therapy, laboratory findings within 48 hours of admission, radiologic findings including chest X-ray and computed tomography (CT), medications (remdesivir, antibiotics and steroids), and clinical outcomes (transfer to other hospitals, readmission or mortality). The duration of admission and from admission to oxygen supply (overall and within 3 days), length of time to pneumonia detection, National Early Warning Score-2 (NEWS-2),
11 and National Institute of Allergy and Infectious Disease Ordinal Scale (NIAID-OS)
12 score were calculated. Pneumonic infiltration on chest radiography was divided into unilateral and bilateral infiltrates.
Definition
The duration from onset of illness to discharge was calculated from the first date of symptoms or positive RT-PCR without symptoms to discharge, transfer, or death at the study hospitals. New steroid treatment used for only COVID-19 during admission was analyzed, and other steroid treatments for underlying diseases, septic shock or acute respiratory distress syndrome not related to COVID-19 were excluded. We defined the use of dexamethasone 6 mg or an equivalent dose of other steroids within 10 days as standard use (standard group) based on current guidelines.
567 A dose deviation was defined as a higher or lower dose, and the use of steroids > 10 days was defined as longer use.
Statistical analysis
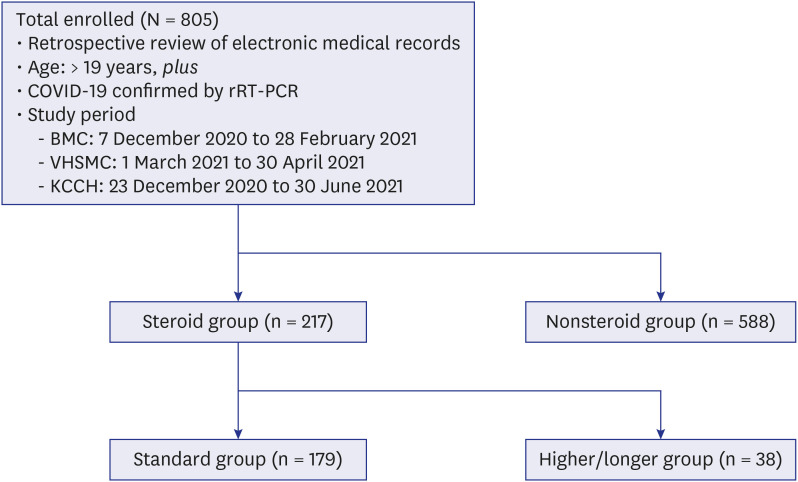
The subjects were divided into a steroid group and a nonsteroid group, and the steroid group was then subdivided into a standard group and a higher/longer group (
Fig. 1). To compare categorical variables, the χ
2 test or Fisher’s exact test was used. Student’s
t-test was used to compare continuous variables. Logistic regression was used to calculate odds ratio (OR) and associated 95% confidence interval (CI). Variables were analyzed by backward stepwise logistic regression for the multivariable analysis. Variables were removed using a
P value of 0.05 as the cutoff value. In all analyses, a
P value < 0.05 was considered statistically significant. Statistical analysis was performed using IBM SPSS Statistics (ver. 28.0; IBM, Armonk, NY, USA).
Fig. 1
Study design.
COVID-19 = coronavirus disease 2019, rRT-PCR = real-time reverse transcription-polymerase chain reaction, BMC = Boramae Medical Center, VHSMC = Veterans Health Service Medical Center, KCCH = Korea Cancer Center Hospital.
Ethics statement
This study protocol was reviewed and approved by the Institutional Review Board (IRB) of BMC (No. 20-2021-53) and VHSMC (No. 2022-01-034), and informed consent was waived by the IRB because of the retrospective nature of the study. All personal identifiers were anonymized for confidentiality before data processing. This study was in compliance with the Helsinki Declaration.
RESULTS
Baseline characteristics of COVID-19 patients with steroid use
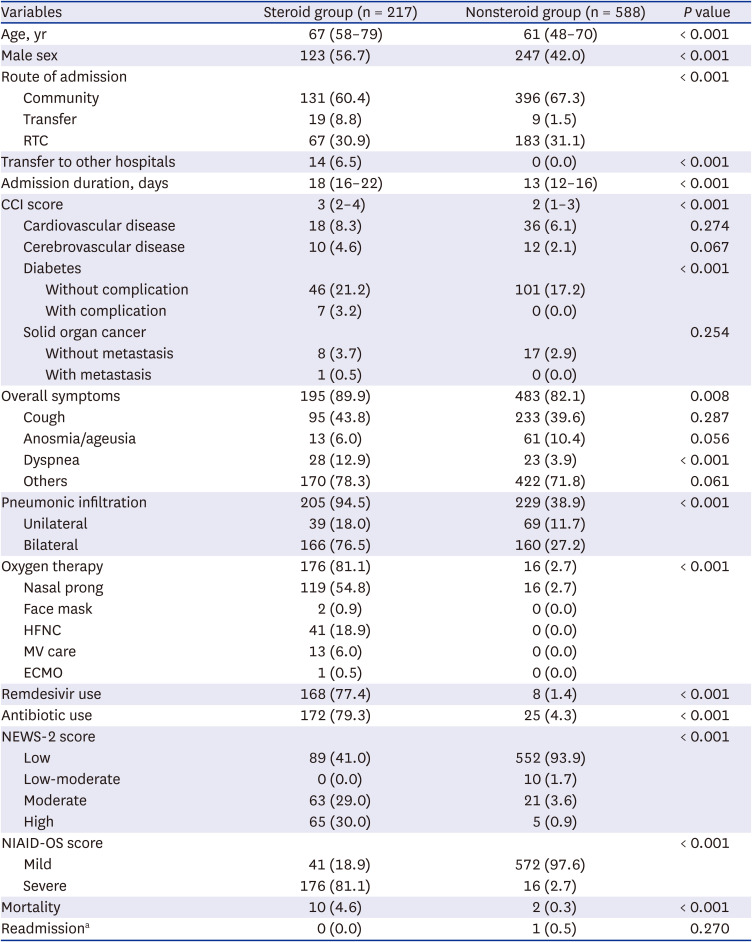
A total of 805 patients were enrolled, and 217 (27.0%) patients were treated with steroids (
Table 1). The steroid group in comparison with the nonsteroid group was older (median 67 vs. 61 years), had more males (56.7% vs. 42.0%) and had a higher CCI score (median 3 vs. 2), especially high frequency of diabetes regardless of complication (24.4% vs. 17.2%). The steroid group stayed longer in the hospital (median 18 vs. 13 days) and was transferred to other hospitals more frequently (6.5% vs. 0.0%) for ventilator care. Regarding clinical manifestations, cough was most frequent (43.8% vs. 39.6%) but was not significantly different between the groups. Dyspnea (12.9% vs. 3.9%) and radiologic evidence of pneumonia (94.5% vs. 38.9%), especially bilateral pneumonia (76.5% vs. 27.2%), were more frequent in the steroid group. The steroid group showed a disproportionately higher rate of oxygen therapy (81.1% vs. 2.7%), more concomitant use of remdesivir (77.4% vs. 1.4%) and more use of antibiotics (79.3% vs. 4.3%). The frequent types of oxygen supply used in the steroid group were nasal prong (54.8%) and high-flow nasal cannula (HFNC, 18.9%). The severity indices of high risk according to NEWS-2 score (30.0% vs. 0.9%) and severe risk according to the NIAID-OS score (81.1% vs. 2.7%) were significantly high in the steroid group. In-hospital mortality was 4.6% in the steroid group and 0.3% in the nonsteroid group. Although combined bacterial pneumonia was not clearly distinguishable in most fatal patients, other serious infectious complications including cytomegalovirus diseases, invasive aspergillosis, or serious bacterial coinfection were not documented in both two groups.
Table 1
Baseline characteristics of COVID-19 patients with and without steroid use (N = 805)
|
Variables |
Steroid group (n = 217) |
Nonsteroid group (n = 588) |
P value |
|
Age, yr |
67 (58–79) |
61 (48–70) |
< 0.001 |
|
Male sex |
123 (56.7) |
247 (42.0) |
< 0.001 |
|
Route of admission |
|
|
< 0.001 |
|
Community |
131 (60.4) |
396 (67.3) |
|
Transfer |
19 (8.8) |
9 (1.5) |
|
RTC |
67 (30.9) |
183 (31.1) |
|
Transfer to other hospitals |
14 (6.5) |
0 (0.0) |
< 0.001 |
|
Admission duration, days |
18 (16–22) |
13 (12–16) |
< 0.001 |
|
CCI score |
3 (2–4) |
2 (1–3) |
< 0.001 |
|
Cardiovascular disease |
18 (8.3) |
36 (6.1) |
0.274 |
|
Cerebrovascular disease |
10 (4.6) |
12 (2.1) |
0.067 |
|
Diabetes |
|
|
< 0.001 |
|
|
Without complication |
46 (21.2) |
101 (17.2) |
|
|
With complication |
7 (3.2) |
0 (0.0) |
|
Solid organ cancer |
|
|
0.254 |
|
|
Without metastasis |
8 (3.7) |
17 (2.9) |
|
|
With metastasis |
1 (0.5) |
0 (0.0) |
|
Overall symptoms |
195 (89.9) |
483 (82.1) |
0.008 |
|
Cough |
95 (43.8) |
233 (39.6) |
0.287 |
|
Anosmia/ageusia |
13 (6.0) |
61 (10.4) |
0.056 |
|
Dyspnea |
28 (12.9) |
23 (3.9) |
< 0.001 |
|
Others |
170 (78.3) |
422 (71.8) |
0.061 |
|
Pneumonic infiltration |
205 (94.5) |
229 (38.9) |
< 0.001 |
|
Unilateral |
39 (18.0) |
69 (11.7) |
|
Bilateral |
166 (76.5) |
160 (27.2) |
|
Oxygen therapy |
176 (81.1) |
16 (2.7) |
< 0.001 |
|
Nasal prong |
119 (54.8) |
16 (2.7) |
|
Face mask |
2 (0.9) |
0 (0.0) |
|
HFNC |
41 (18.9) |
0 (0.0) |
|
MV care |
13 (6.0) |
0 (0.0) |
|
ECMO |
1 (0.5) |
0 (0.0) |
|
Remdesivir use |
168 (77.4) |
8 (1.4) |
< 0.001 |
|
Antibiotic use |
172 (79.3) |
25 (4.3) |
< 0.001 |
|
NEWS-2 score |
|
|
< 0.001 |
|
Low |
89 (41.0) |
552 (93.9) |
|
Low-moderate |
0 (0.0) |
10 (1.7) |
|
Moderate |
63 (29.0) |
21 (3.6) |
|
High |
65 (30.0) |
5 (0.9) |
|
NIAID-OS score |
|
|
< 0.001 |
|
Mild |
41 (18.9) |
572 (97.6) |
|
Severe |
176 (81.1) |
16 (2.7) |
|
Mortality |
10 (4.6) |
2 (0.3) |
< 0.001 |
|
Readmissiona
|
0 (0.0) |
1 (0.5) |
0.270 |
Analysis of steroid use
Dosing and duration of steroid use
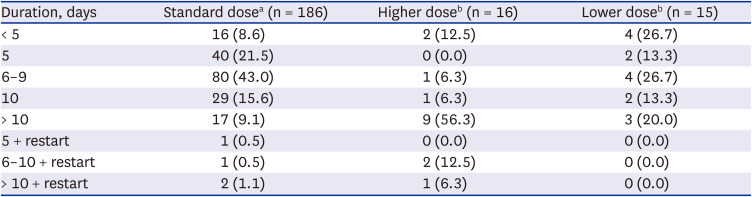
Most patients (179/217, 82.5%) in the steroid group received a standard or lower dose of steroids within ten days (
Table 2). Among the patients receiving standard dosing (186/217, 85.7%), 11.3% (21/186) received steroids over ten days or restarted steroids. However, in the patients with higher dosing (16/217, 7.4%), 75.0% (12/16) received steroids over ten days or restarted steroids.
Table 2
Dosage and duration of steroid treatment in COVID-19 patients (N = 217)
|
Duration, days |
Standard dosea (n = 186) |
Higher doseb (n = 16) |
Lower doseb (n = 15) |
|
< 5 |
16 (8.6) |
2 (12.5) |
4 (26.7) |
|
5 |
40 (21.5) |
0 (0.0) |
2 (13.3) |
|
6–9 |
80 (43.0) |
1 (6.3) |
4 (26.7) |
|
10 |
29 (15.6) |
1 (6.3) |
2 (13.3) |
|
> 10 |
17 (9.1) |
9 (56.3) |
3 (20.0) |
|
5 + restart |
1 (0.5) |
0 (0.0) |
0 (0.0) |
|
6–10 + restart |
1 (0.5) |
2 (12.5) |
0 (0.0) |
|
> 10 + restart |
2 (1.1) |
1 (6.3) |
0 (0.0) |
Higher or longer use of steroids
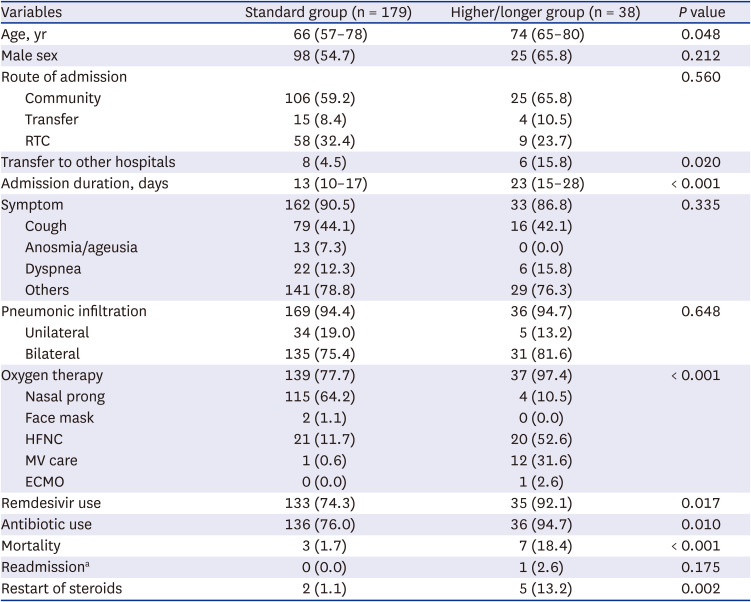
Among 217 patients in the steroid group, 38 (17.5%) patients received higher doses or longer duration of steroids (
Table 3). The higher/longer group was older (median 74 vs. 66 years), had a longer admission duration (median 23 vs. 13 days) and was more frequently transferred to other hospitals (15.8% vs. 4.5%) than the standard group. Oxygen therapy was more frequent in the higher/longer group (37/38, 97.4% vs. 139/179, 77.7%), especially in the form of HFNC (20/38, 52.6% vs. 21/179, 11.7%). Mechanical ventilator (MV) care (31.6% vs. 0.6%) and extracorporeal membrane oxygenation (ECMO) (2.6% vs. 0.0%) were applied more frequently, and the proportion of use of remdesivir (92.1% vs. 74.3%) or antibiotics (94.7% vs. 76.0%) and restart of steroids (13.2% vs. 1.1%) was higher in the higher/longer group than in the standard group.
Table 3
Characteristics of COVID-19 patients with higher or longer use of steroids (N = 217)
|
Variables |
Standard group (n = 179) |
Higher/longer group (n = 38) |
P value |
|
Age, yr |
66 (57–78) |
74 (65–80) |
0.048 |
|
Male sex |
98 (54.7) |
25 (65.8) |
0.212 |
|
Route of admission |
|
|
0.560 |
|
Community |
106 (59.2) |
25 (65.8) |
|
Transfer |
15 (8.4) |
4 (10.5) |
|
RTC |
58 (32.4) |
9 (23.7) |
|
Transfer to other hospitals |
8 (4.5) |
6 (15.8) |
0.020 |
|
Admission duration, days |
13 (10–17) |
23 (15–28) |
< 0.001 |
|
Symptom |
162 (90.5) |
33 (86.8) |
0.335 |
|
Cough |
79 (44.1) |
16 (42.1) |
|
Anosmia/ageusia |
13 (7.3) |
0 (0.0) |
|
Dyspnea |
22 (12.3) |
6 (15.8) |
|
Others |
141 (78.8) |
29 (76.3) |
|
Pneumonic infiltration |
169 (94.4) |
36 (94.7) |
0.648 |
|
Unilateral |
34 (19.0) |
5 (13.2) |
|
Bilateral |
135 (75.4) |
31 (81.6) |
|
Oxygen therapy |
139 (77.7) |
37 (97.4) |
< 0.001 |
|
Nasal prong |
115 (64.2) |
4 (10.5) |
|
Face mask |
2 (1.1) |
0 (0.0) |
|
HFNC |
21 (11.7) |
20 (52.6) |
|
MV care |
1 (0.6) |
12 (31.6) |
|
ECMO |
0 (0.0) |
1 (2.6) |
|
Remdesivir use |
133 (74.3) |
35 (92.1) |
0.017 |
|
Antibiotic use |
136 (76.0) |
36 (94.7) |
0.010 |
|
Mortality |
3 (1.7) |
7 (18.4) |
< 0.001 |
|
Readmissiona
|
0 (0.0) |
1 (2.6) |
0.175 |
|
Restart of steroids |
2 (1.1) |
5 (13.2) |
0.002 |
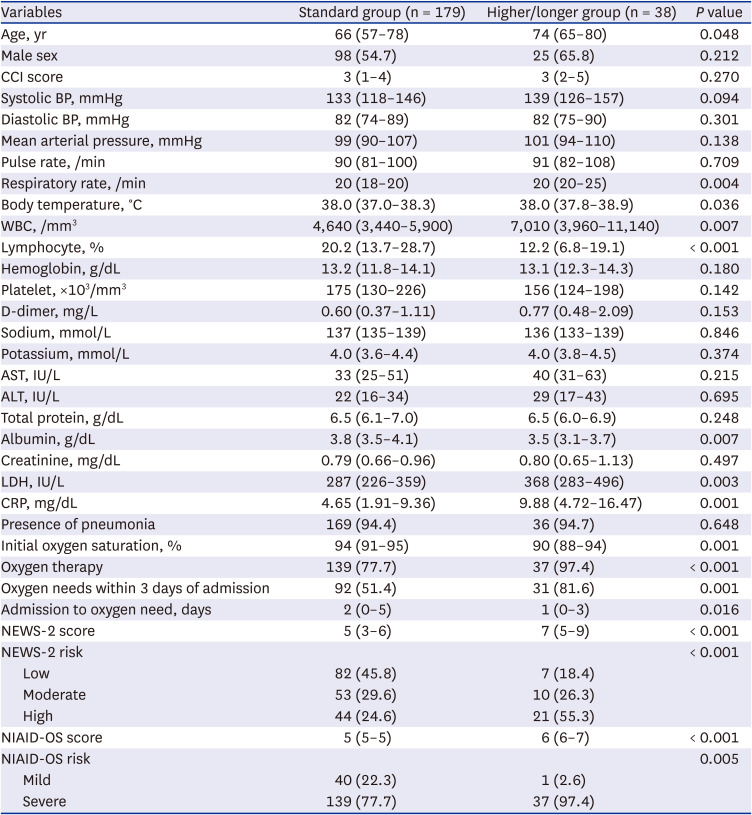
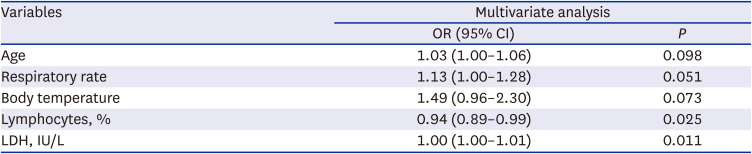
Risk factors for higher or longer use of steroids
Univariable analysis showed that the following factors within 24 hours after admission were significantly associated with the higher/longer group: older age (median 74 years), tachypnea (median 20/min), fever (median 38.0°C), lymphopenia (median 11.6%), overall oxygen need within three days from admission (81.6%), shorter duration from admission to oxygen need (median 1 day), higher risk according to NEWS-2 score (median 7) or NIAID-OS score (median 6), higher white blood cell count (median 7,010/mm
3), lactate dehydrogenase (LDH) level (median 368 IU/L) or C-reactive protein (median 9.88 mg/dL), and lower level of albumin (median 3.5 g/dL) or oxygen saturation (median 90%) (
Table 4). Multivariable analysis showed that lymphopenia (adjusted odds ratio [aOR], 0.94; 95% CI, 0.89–0.99;
P = 0.025), and higher LDH (aOR, 1.00; 95% CI, 1.00–1.01;
P = 0.011) were independent risk factors for higher or longer steroid use (
Table 5).
Table 4
Univariable analysis of risk factors for COVID-19 patients with higher doses or longer durations of steroid use (N = 217)
|
Variables |
Standard group (n = 179) |
Higher/longer group (n = 38) |
P value |
|
Age, yr |
66 (57–78) |
74 (65–80) |
0.048 |
|
Male sex |
98 (54.7) |
25 (65.8) |
0.212 |
|
CCI score |
3 (1–4) |
3 (2–5) |
0.270 |
|
Systolic BP, mmHg |
133 (118–146) |
139 (126–157) |
0.094 |
|
Diastolic BP, mmHg |
82 (74–89) |
82 (75–90) |
0.301 |
|
Mean arterial pressure, mmHg |
99 (90–107) |
101 (94–110) |
0.138 |
|
Pulse rate, /min |
90 (81–100) |
91 (82–108) |
0.709 |
|
Respiratory rate, /min |
20 (18–20) |
20 (20–25) |
0.004 |
|
Body temperature, °C |
38.0 (37.0–38.3) |
38.0 (37.8–38.9) |
0.036 |
|
WBC, /mm3
|
4,640 (3,440–5,900) |
7,010 (3,960–11,140) |
0.007 |
|
Lymphocyte, % |
20.2 (13.7–28.7) |
12.2 (6.8–19.1) |
< 0.001 |
|
Hemoglobin, g/dL |
13.2 (11.8–14.1) |
13.1 (12.3–14.3) |
0.180 |
|
Platelet, ×103/mm3
|
175 (130–226) |
156 (124–198) |
0.142 |
|
D-dimer, mg/L |
0.60 (0.37–1.11) |
0.77 (0.48–2.09) |
0.153 |
|
Sodium, mmol/L |
137 (135–139) |
136 (133–139) |
0.846 |
|
Potassium, mmol/L |
4.0 (3.6–4.4) |
4.0 (3.8–4.5) |
0.374 |
|
AST, IU/L |
33 (25–51) |
40 (31–63) |
0.215 |
|
ALT, IU/L |
22 (16–34) |
29 (17–43) |
0.695 |
|
Total protein, g/dL |
6.5 (6.1–7.0) |
6.5 (6.0–6.9) |
0.248 |
|
Albumin, g/dL |
3.8 (3.5–4.1) |
3.5 (3.1–3.7) |
0.007 |
|
Creatinine, mg/dL |
0.79 (0.66–0.96) |
0.80 (0.65–1.13) |
0.497 |
|
LDH, IU/L |
287 (226–359) |
368 (283–496) |
0.003 |
|
CRP, mg/dL |
4.65 (1.91–9.36) |
9.88 (4.72–16.47) |
0.001 |
|
Presence of pneumonia |
169 (94.4) |
36 (94.7) |
0.648 |
|
Initial oxygen saturation, % |
94 (91–95) |
90 (88–94) |
0.001 |
|
Oxygen therapy |
139 (77.7) |
37 (97.4) |
< 0.001 |
|
Oxygen needs within 3 days of admission |
92 (51.4) |
31 (81.6) |
0.001 |
|
Admission to oxygen need, days |
2 (0–5) |
1 (0–3) |
0.016 |
|
NEWS-2 score |
5 (3–6) |
7 (5–9) |
< 0.001 |
|
NEWS-2 risk |
|
|
< 0.001 |
|
Low |
82 (45.8) |
7 (18.4) |
|
Moderate |
53 (29.6) |
10 (26.3) |
|
High |
44 (24.6) |
21 (55.3) |
|
NIAID-OS score |
5 (5–5) |
6 (6–7) |
< 0.001 |
|
NIAID-OS risk |
|
|
0.005 |
|
Mild |
40 (22.3) |
1 (2.6) |
|
Severe |
139 (77.7) |
37 (97.4) |
Table 5
Multivariable analysis of risk factors for COVID-19 patients with higher doses or longer durations of steroid use
|
Variables |
Multivariate analysis |
|
OR (95% CI) |
P
|
|
Age |
1.03 (1.00–1.06) |
0.098 |
|
Respiratory rate |
1.13 (1.00–1.28) |
0.051 |
|
Body temperature |
1.49 (0.96–2.30) |
0.073 |
|
Lymphocytes, % |
0.94 (0.89–0.99) |
0.025 |
|
LDH, IU/L |
1.00 (1.00–1.01) |
0.011 |
DISCUSSION
Among severe or critically ill patients hospitalized with COVID-19, dexamethasone 6 mg for 10 days (or until discharge) or an equivalent glucocorticoid dose is recommended in the current treatment guidelines for COVID-19.
567 Equivalent total daily doses of alternative glucocorticoids to dexamethasone 6 mg daily are methylprednisolone 32 mg and prednisone 40 mg. Patients in the steroid group in our study had characteristic variables indicating severe disease, and they were in line with the current recommendation of steroid use for severe or critical illness. Older age, comorbidities, initial symptoms and direct radiologic evidence indicating severe pneumonia and the use of oxygen therapy were such variables (
Table 1). Remdesivir was concomitantly used in 77.4% of patients, and national guidelines and occasional shortages of the drug may explain the incompletely concordant use of remdesivir and steroids. Oxygen therapy was needed in 81.1% of patients in the steroid group, and 54.8% were with nasal cannula, 18.9% with HFNC, 0.9% with face mask and 6.5% with invasive ventilation. Face masks were rarely used, and HFNC was the predominant tool for high-flow oxygen therapy and might reduce the burden of invasive ventilation. The transfer rate to other hospitals was 6.5%, and the lack of invasive ventilation was a main reason. In-hospital mortality was 4.6%. In a single-center study on steroid use in 200 COVID-19 patients, age over 70 years and hypertension were the predominant risk factors for mortality.
13 The 28-day mortality was higher in patients with steroid use (19.5%) than in those without steroid use (3.9%) in the study. While the reduction in 28-day mortality by steroid use was attested by a previous study,
9 the higher mortality of COVID-19 patients with steroid use reflected the severity of the study population in nature. The high rate of antibiotic use in our study, 79.3%, is also of particular note compared with 4.3% in the nonsteroid group.
Among the steroid group, 77.0% were treated with both a standard dose and ≤ 10 days of steroids, 6.9% with a lower dose, and 16.1% with a higher dose or > 10 days of steroids. Early termination of steroids associated with transfer to other hospitals was observed in some patients, and early end of steroids by death was not seen. This suggests that most eligible patients for current steroid treatment criteria had a favorable response, but there were other groups who required a lower dose of steroids or who required a longer use of steroids or additional therapeutic modalities. Strategies and information on surrogate markers to tailor the optimal duration and dose of steroids are needed.
In COVID-19 patients with higher doses or longer duration of steroid use, characteristics similar to those seen in the steroid group were observed: older age, prolonged duration of hospital stay, higher transfer rate to other hospitals and increased mortality (
Table 3). Independent risk factors for higher doses or longer duration of steroid use were lymphopenia and higher level of LDH within 2 days of admission (
Table 5). Although current guidelines recommend steroid use for 10 days or until discharge,
567 those variables may be used to predict the possibility of higher or longer use of steroids in severe COVID-19 patients. Antibiotics were used in 94.7% of the higher/longer group.
Antibiotic use in COVID-19 patients is an uncertain active issue in terms of the degree of bacterial coinfection and antibiotic stewardship. Recent studies showed that 7.2–8.6% of COVID-19 patients had community- or hospital-acquired bacterial coinfections, and superinfections had worse outcomes. The prevalence of antibiotic prescriptions was 74.6%, which was disproportionately higher than the estimated coinfections.
1415 A strategy for antimicrobial stewardship for COVID-19 using C-reactive protein and procalcitonin was suggested.
16
There were some limitations in our study. First, although we enrolled the subjects from three study hospitals and the number of subjects was not so small, they did not represent the Korean situation as a whole. However, as those were typical COVID-19 hospitals, the result may be extrapolated to other COVID-19 hospitals in Korea. Second, the subjects from each study hospital were enrolled in different periods because of hospital differences in their initiation of operations for COVID-19 care and inpatient capacity. COVID-19 vaccination for the general population in Korea began after April 2021, and the number of patients enrolled in the early period of vaccination was 143 (17.8%). The effect of vaccination on the clinical outcome might be limited, and our study covered largely unvaccinated COVID-19 patients. Third, the dominant genotype has changed over time during the pandemic, and our descriptive result was from a non-omicron period. The results may not be applicable to different virologic backgrounds. However, steroids are still an important therapeutic option, and our results can be used as a reference to understand the situation of steroid use in COVID-19 patients in our community. Lastly, as our study retrospectively described the real practices and each physician in charge might use their own criteria in decision to choose the dose and duration of steroids, we could not objectively conclude whether the higher dose or longer duration of steroids would lead to better outcomes nor the risk factors identified here would actually predict the need of extra-steroids. Well-designed comparative studies would be needed for the better answer. Our study showed the possible margin to be intervened.
In summary, we described the clinical practice of steroid use in severe COVID-19 patients in Korean hospitals. The dose and duration of steroids in line with current guidelines could be applied in 82.5% of patients, but the strategies using surrogate markers may need to be tailored to outliers. Initial presentation with lymphopenia or high level of LDH was associated with a higher dose or longer use of steroids in severe COVID-19 patients.









 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download