This article has been
cited by other articles in ScienceCentral.
Abstract
Background
The rapid urease test (RUT) is a major diagnostic tool for detecting Helicobacter pylori infection. This study aimed to establish an objective method for measuring the color changes in the RUT kit to improve the test’s diagnostic accuracy.
Methods
A UV-visible spectrophotometer was selected as the colorimeter; experiments were conducted in three stages to objectively identify the color changes in the RUT kit.
Results
First, the urea broth solution showed an identifiable color change from yellow to red as the pH increased by 0.2. The largest transmittance difference detected using the UV-visible spectrophotometer was observed at a 590-nm wavelength. Second, the commercialized RUT kit also showed a gradual color change according to the pH change detected using the UV-visible spectrophotometer. Third, 13 cases of negative RUT results with a biopsy specimen and 16 of positive RUT results were collected. The transmittance detected using the UV-visible spectrophotometer showed a clear division between the positive and negative RUT groups; the largest difference was observed at a 559-nm wavelength. The lowest transmittance in the negative RUT group was 64, while the highest in the positive RUT group was 56, at the 559-nm wavelength. The UV-visible spectrophotometry reading showed a consistency of 92.7% compared with that of manual reading.
Conclusion
A transmittance of 60 at a 559-nm wavelength detected using UV-visible spectrophotometer can be used as a cutoff value for interpreting RUT results; this will help develop an automatic RUT kit reader with a high accuracy.
Keywords: Helicobacter pylori, Rapid Urease Test, Diagnosis, Spectrophotometer
INTRODUCTION
Helicobacter pylori is a major human pathogen detected in individuals of all ages worldwide, which colonizes the gastric mucosa, leading to atrophic gastritis, gastroduodenal ulcer, and gastric cancer.
123 An accurate diagnostic tool is necessary for
H. pylori infection treatment; however, to date, no gold standard diagnostic method has been established. The current diagnostic tools are divided into non-invasive, including the urea breath test (UBT) and serology analysis, and invasive methods, including the rapid urease test (RUT), histopathology, culture, and polymerase chain reaction (PCR). The choice of diagnostic method depends on the clinical situation, considering the sensitivity, specificity, availability, cost, and rapidity. Specifically, the RUT has the advantages of yielding results in a short period and of being cheap and simple to use, with a sensitivity of 75–100% and a specificity of 84–100%.
45
The foremost RUT is a diagnostic tool composed of an agar gel containing urea and a pH indicator. It detects the urease of
H. pylori, which produces ammonia and increases the pH, subsequently inducing a color change in the kit.
6 The RUT kit changes color from yellow to red when
H. pylori is present; this color change is read visually by an interpreter (usually a nurse or a doctor) after a certain period, as recommended in the kit. This study aimed to objectively measure the color changes in the RUT to increase the accuracy, sensitivity, and specificity of the test.
METHODS
Three stages of experiments were conducted to objectively measure the color changes in the RUT (kit; ChongKunDAng BIO HP Kit), and a UV-visible spectrophotometer was selected as the colorimeter.
Color measurement of the urea broth solution and RUT
Urea broth (1.9 g) was dissolved in 50 mL of deionized water at room temperature (22 ± 2°C) for 5 minutes. The pH of the solution was increased up to 8.4 at an interval of 0.2 using a diluted ammonia solution (0.1M). The urea broth solutions (2 mL) were placed in cuvettes, and the transmittance at wavelengths ranging from 380 to 650 nm was measured using the UV-visible spectrophotometer.
To confirm the pH effects on the RUT results, the ammonia solution was diluted in deionized water as a function of pH, and 5 μL of ammonia solution was then dropped into the RUT kit. After 30 minutes, the transmittance of the RUT was measured at wavelengths ranging from 380 to 650 nm using the UV-visible spectrophotometer with an integrating sphere. The stomach biopsy specimen was placed in the RUT kit for 2 hours, and the transmittance was measured in the same manner.
Analysis of the RUT kits using the UV-visible spectrophotometer
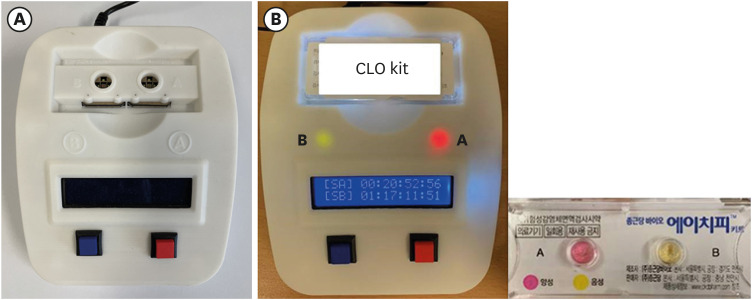
The color changes in the RUT kit in clinical practice were compared by multiple individuals and the UV-visible spectrophotometer. A total of two samples of stomach tissue were obtained from the antrum and body and were separately placed into RUT kits A and B; thereafter, the RUT kit was immediately placed on the UV-visible spectrophotometer to confirm positivity through a color change over time. Moreover, the time taken for the color to change was assessed; it was identified as negative when there was no color change for 24 hours (
Fig. 1). In total, 100 RUT kit color changes in 50 patients were compared. The accordance and discrepancy in the readings obtained by the different investigators or using the UV-visible spectrophotometer were investigated. The discordant cases were reconfirmed with additional UBT and culture which is considered as a gold standard method for diagnosis of
H. pylori infection.
Fig. 1
Reading of the color change in the RUT kits using the UV-visible spectrophotometer. (A) UV-visible spectrophotometer model. (B) Positivity and time record for the color change in RUT kits A and B. A (+): 20 hours 52 minutes 56 seconds; B (−): 1 day 17 hours 11 minutes 51 second.
RUT = rapid urease test.
Ethics statement
The Institutional Review Board of the Asan Medical Center approved this study (IRB approval number: 2021-1629).
RESULTS
Color measurement of the urea broth solution
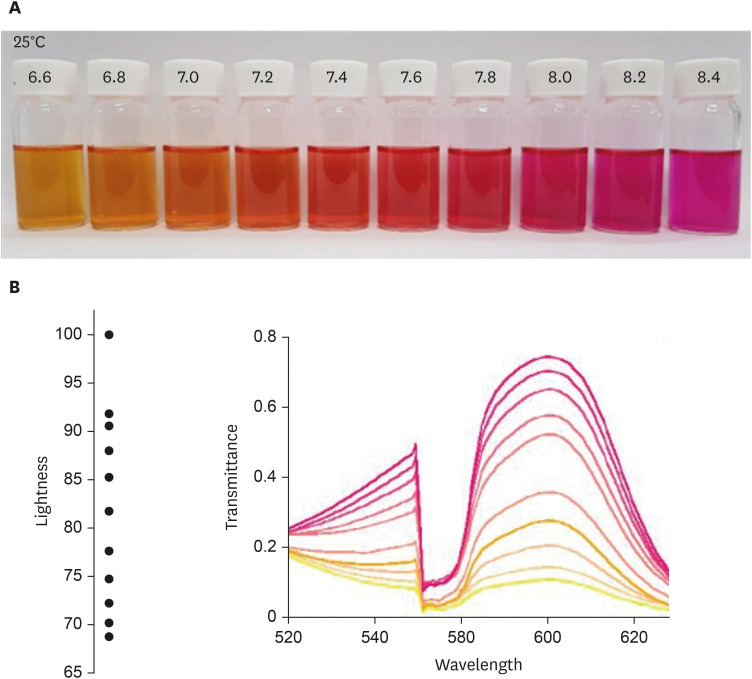
The urea broth solution was added to phenol red, which increased the pH and subsequently induced a color change from yellow to red. As the pH increased by 0.2 from 6.2, the redness gradually deepened (
Fig. 2A). The UV-visible spectrophotometer was used to measure the color spectrum; the transmittance was measured differently according to the wavelength (
Fig. 2B). The largest transmittance difference detected using the UV-visible spectrophotometer was observed at a wavelength of 590 nm.
Fig. 2
Color spectrum according to the pH change in the urea broth solution detected using the UV-visible spectrophotometer. (A) Color change in the urea broth solution according to the pH change. (B) Color change in the urea broth solution according to the pH change detected using the UV-visible spectrophotometer.
Color measurement of the RUT kit
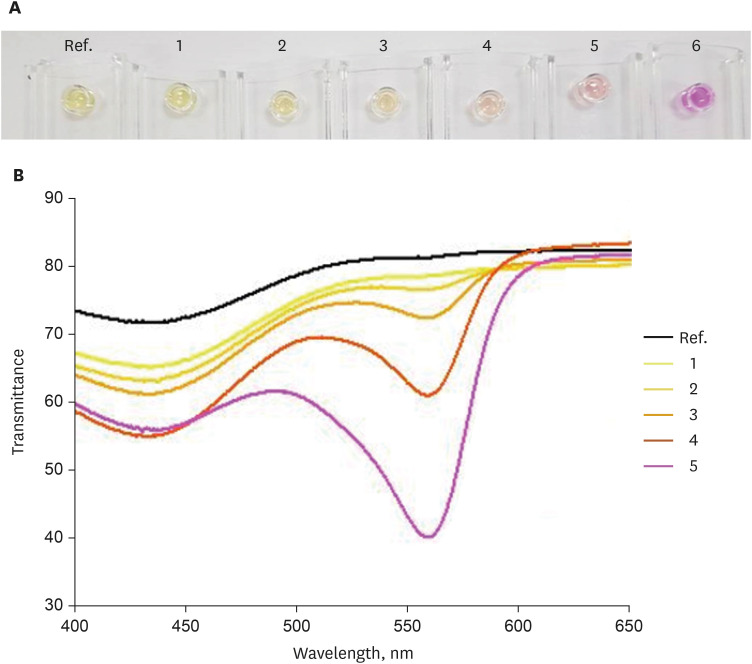
The RUT kit showed a gradual color change in association with the pH change (
Fig. 3A). The largest transmittance difference was observed at a wavelength of 560 nm (
Fig. 3B).
Fig. 3
Color spectrum according to the pH change in the RUT kit detected using the UV-visible spectrophotometer. (A) Color change in the RUT kit according to the pH change. (B) Color change in the RUT kit according to the pH change detected using the UV-visible spectrophotometer.
pH: 1 = 7.3, 2 = 9.9, 3 = 10.2, 4 = 10.3, 5 = 10.5, 6 = 10.9.
RUT = rapid urease test.
RUT kit with the stomach biopsy specimen
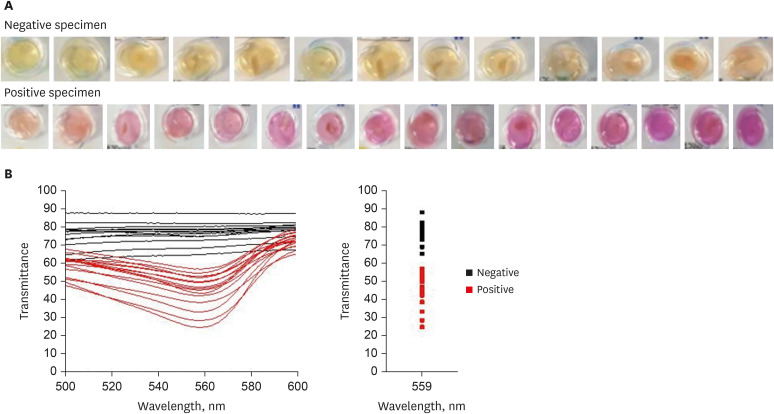
The color of the RUT kit changed from yellow to red when the stomach biopsy specimen was positive for
H. pylori. However, the biopsy tissue or blood can also make the RUT kit color reddish. A total of 13 negative and 16 positive RUT kit results were collected with various sizes of biopsy specimens and blood stains of various sizes (
Fig. 4A). The transmittance detected using the UV-visible spectrophotometer showed a clear division between the positive and negative RUT groups, and the largest difference was observed at a wavelength of 559 nm (
Fig. 4B). The lowest transmittance in the negative RUT group was 64, while the highest transmittance in the positive RUT group was 56, at a wavelength of 559 nm.
Fig. 4
Color spectrum in the RUT kit with the gastric biopsy specimen detected using the UV-visible spectrophotometer. (A) Color change in the RUT kit with the gastric biopsy specimen. (B) Color change in the RUT kit with the gastric biopsy specimen detected using the UV-visible spectrophotometer.
RUT = rapid urease test.
Verification of the accuracy of the UV-visible spectrophotometer
A total of 96 RUT kit color changes in 48 patients, excluding two whose results were ambiguous, were compared. Of these, 92.7% (89/96) of the manual and UV-visible spectrophotometry readings was consistent, and 7.3% (7/96) was discordant (
Supplementary Table 1). Seven discordant cases were compared with additional test results including those of the UBT and culture. In 2 cases with H. pylori infection (culture positive), one case was negative by manual reading and the other was negative by UV-visible spectrophotometry reading. In 4 cases not expected to be infected with
H. pylori (both culture and UBT negative), 3 cases showed positive by manual reading and 1 case showed positive by UV-visible spectrophotometry reading.
DISCUSSION
The RUT is one of the major methods used for detecting
H. pylori infection. However, the RUT alone is not the gold standard for its diagnosis, and recent studies have recommended the use of two or three available techniques, such as the UBT, culture, and PCR, to diagnose
H. pylori infection. A previous study has found a negative RUT result to be culture-positive in 18.3% of cases among 544 RUT results, and a positive RUT result was culture-negative in 16% of cases.
7 Although the RUT has shown high sensitivity and specificity, there is a need to improve its accuracy. The RUT results can be affected by exogenous factors, such as density of bacteria, atrophy of the gastric mucosa, and recent use of medications, including antibiotics and proton pump inhibitors.
48910
In addition to patient-related factors, a major factor in interpreting the results is related to the accuracy of the examination. It is important to read the RUT results at the time recommended in the kit.
11 However, in practice, it is difficult to read all RUT results at regular time intervals because of labor shortage or large case volumes. Moreover, the reference color for a positive result should be unambiguous. Results with a slight color change are identified via the reader’s opinion, and there are some cases where readings may vary among individuals. Therefore, an automatic RUT kit reader that is capable of reading the results at an appropriate time and reducing human error is required. A positive index color must then be determined; therefore, we attempted to measure the color changes in the RUT kit objectively using the UV-visible spectrophotometer.
In this study, the 559-nm wavelength showed a clear difference in transmittance between the negative and positive RUT groups. Moreover, the UV-visible spectrophotometry reading showed a consistency of 92.7% compared with manual reading in clinical practice.
The novel device used in our study has several limitations. First, it can measure only one RUT kit at a time. It should be addressed before the commercialization of this device. Second, after completing the RUT kit reading, an individual should check and record the result inconveniently. However, if the results can be set to be linked to the computer when the analysis is complete, it is expected that the diagnostic process can also be shortened. A large-scale randomized prospective study is needed to prove that this cutoff color is effective for accurately diagnosing H. pylori infection and for confirming the feasibility of the use of the UV-visible spectrophotometer.
In conclusion, the transmittance detected using the UV-visible spectrophotometer differed between the positive and negative RUT groups at a wavelength of 559 nm. The lowest transmittance in the negative RUT group was 64, while the highest in the positive RUT group was 56. Therefore, a transmittance of 60 at a wavelength 559 nm may be a potential cutoff value for interpreting RUT results. This finding could serve as a basis for developing an automatic RUT kit reader to improve the diagnostic accuracy.








 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download