Gastric cancer, which is one of the most common cancers and the fourth leading cause of cancer death, is a major health problem worldwide.
1 Gastric cancer often has a poor prognosis as the diagnosis can be delayed due to the lack of clinical signs until it reaches an advanced stage, leading to incurable, progressive disease in many cases.
2 Recent advances in molecular genetics research technology have enabled detailed studies of the molecular characteristics of gastric cancer, increasing the possibility of predicting the optimal treatment for individual patients.
3 However, lack of useful preclinical models hampers the development of targeted anti-cancer drugs, which is crucial for precision medicine. Organoids are three-dimensional (3D) cell culture systems with organ-like structures and functions that have been generated from primary tissues or embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), or adult stem cells (ASCs).
4 As patient-derived organoids (PDOs) can maintain the phenotypic and molecular characteristics of the primary cancer tissues including the genetic alterations, PDO models can be used to predict therapeutic response to specific drugs. Construction of organoid biobanks could be useful in suggesting new therapeutic strategies for gastric cancer, and these PDO models can fill the gap between cancer genetics and phenotypes, which are essential for application as a method for personalized treatment for gastric cancer patients.
5 Recently, there has been increasing attempts to culture cancer organoids using cancer tissues of various organs obtained directly from the surgical specimen of patients.
67 Gastric cancer tissue samples for the development of organoids were also most often obtained from surgically resected specimens. However, in real clinical practice, the majority of patients with advanced disease are not candidates for surgery. Therefore, establishing gastric cancer-PDO using surgical specimens is not often clinically relevant considering the actual clinical setting. On the other hand, tissue acquisition by endoscopic biopsy poses minimal risk to the patient and is a reasonable method to reduce the gap between in vitro cancer models and in vivo drug evaluation. Therefore, we investigated whether a patient-derived gastric cancer organoid could be successfully established with a small amount of cancer tissue obtained by gastroscopy.
Patients who underwent gastroscopy for either initial diagnosis or re-evaluation of gastric cancer at Chug-Ang University Hospital were enrolled. Gastroscopy was performed using a GIF-290 gastroscope (Olympus Co., Tokyo, Japan). After identification of the gastric cancer lesion, standard forcep biopsies using EndoJaw Disposable Biopsy Forceps (Olympus) were performed to obtain 5 pieces of cancer tissues and 3 pieces of normal adjacent tissues (approximately 1–2 mm3 each).
Tissue pieces obtained from gastric cancer patients were immediately put into ice-cold chelating buffer (5.6 mM Na
2HPO
4, 8.0 mM KH
2PO
4, 96.2 mM NaCl, 1.6 mM KCl, 43.4 mM Sucrose, 54.9 mM D-sorbitol, and 0.5 mM DL-dithiothreitol) and directly transported to the laboratory. Tissues were washed, chopped and digested in 5 mL of 0.6 mg/mL collagenase (Sigma-Aldrich, ST. Louis, MO, USA) and 20 μg/mL hyaluronidase (Sigma-Aldrich) in Advanced DMEM/F12 (Gibco-Invitrogen, Carlsbad, CA, USA) along with penicillin, 1 μg/μL primocin, 2.5% fetal bovine serum (FBS), and 10 μM Y-27632 for 1–2 hours at 37°C with occasional gentle shaking. Dissociated cells were transferred to a new tube through a 70-μm cell strainer. The organoids were generated and cultured in the following manner. The dissociated cells were washed and seeded in growth factor and Matrigel (Corning Inc., Corning, NY, USA) along with laboratory prepared organoid culture media (DMEM/F12 media supplemented with antibiotics, Glutamine, HEPES, L-WRN conditioned media for Wnts, R-Spondins and Noggin, as well as B27, N-acetyl cysteine, nicotinamide, hFGF10, Primocin, hEGF, Gastrin, Y-27632, A83-01, and SB202190) (
Fig. 1A). Medium for gastric organoids was replaced every 2–3 days, and passage was performed mainly by dissociation with TrypLE (Thermo Fisher Scientific, Waltham, MA, USA) for 7 minutes at 37°C every 7–14 days, at a ratio of 1:3–1:5. After passaging 3 times, the stocks of organoids were prepared in freezing media containing 40% DMEM/F-12, 50% FBS, 10% dimethyl sulfoxide, and stored in liquid nitrogen after retaining for 2 days at −80°C.
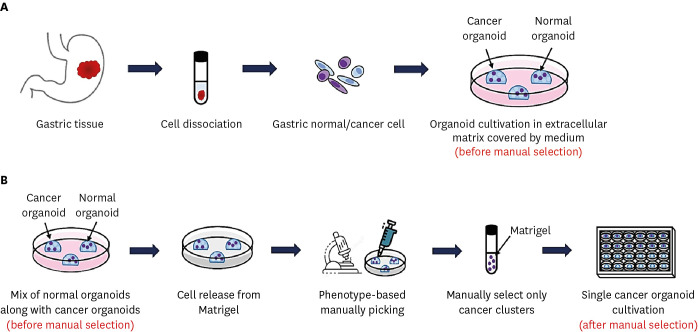 | Fig. 1Schematic diagram of preparation, cultivation and isolation of gastric organoids. (A) The method for generating organoids from endoscopic biopsy. (B) Manual selection procedure to isolate single gastric cancer organoids (pure phenotype) based on morphology from cancer organoid cluster (mixed phenotype).
|
One well-designed method for eliminating normal organoids from mixed cultures and purifying single gastric cancer organoids is manual selection based on the morphology of organoids (
Fig. 1B). If the organoid culture contains mixture of normal organoids and cancer organoids, the cancer organoids can be manually isolated as single organoids. In this case, organoid cultures were dissociated by TrypLE and then embedded in Matrigel as before by manually selecting cancer clusters under a microscope using a 200 μL tip. After that, organoid cultures were resuspended in Matrigel along with organoid culture medium and placed in 96-well plates in very small amounts expected to yield one organoid. After 10–14 days, selection of wells that contain only one organoid was chosen, whereas the wells containing no organoid or more than one organoids were left or discarded. After selecting the obtained single organoid and dissociating it using TrypLE as before, the cells were resuspended in 40 μL of Matrigel, inoculated in a 24-well plate, and then cultured. After one week, multiple pure cancer organoids were achieved from the single organoids. Several rounds of manual selection might be required before a pure population is obtained.
For histopathologic examination and immunohistochemistry (IHC), organoids were fixed with 10% neutral-buffered formalin and embedded with paraffin. Hematoxylin and eosin (H&E), and Periodic acid-Schiff (PAS) staining was performed on Autostainer XL (Leica, Wetzlar, Germany) and Ventana Benchmark Special Stains System (Ventana Medical System, Tucson, AZ, USA), respectively. A polyclonal CEA antibody (Prediluted; Bio SB, Santa Barbara, CA, USA) was used for IHC. IHC was conducted using the Ventana Benchmark XT platform (Ventana Medical System) and the Ultraview DAB detection system (Ventana Medical System).
As a result, 2 tumor organoid culture lines and 5 normal adjacent organoid culture lines were derived from a total of 5 patients. We could detect formation of endoscopic-derived gastric organoids from normal tissues and cancer tissues (
Fig. 2A, top), respectively. In contrast to normal organoids, which exhibit the morphology of cystic structures with lumen, cancer organoids have been reported to exhibit diverse cellular structures.
8 Similarly, in our results, the morphology of normal-PDOs were confirmed as a cystic structure with a thin layer of epithelium and lumen. In contrast, cancer-PDOs (originating from adenocarcinoma) showed an inconsistent grape-like heterogeneous structure. It has been reported that cancer-PDOs exhibit various morphological phenotypes depending on the molecular genotypes.
5 Cancer organoids exhibit a variety of morphologies, such as cystic structures, irregular shapes, wrinkled cell layers, “grape-like” organoids.
8 After three passages of cancer-PDOs, we succeeded in isolating a single cancer organoid from an organoid cluster derived from cancer tissue using the manual selection method (
Fig. 2A; bottom left). The single organoids were then subsequently subcultured, and formation of multiple pure cancer organoids was identified (
Fig. 2A, bottom right). Multiple cancer-PDOs formed small cystic structures after 1 day of initial subculture, and grape-like structures were present after 3 days. In addition, the generated gastric cancer-PDO was continuously cultured through freeze-thaw cycles for more than one year without change in growth behavior or morphological phenotypes.
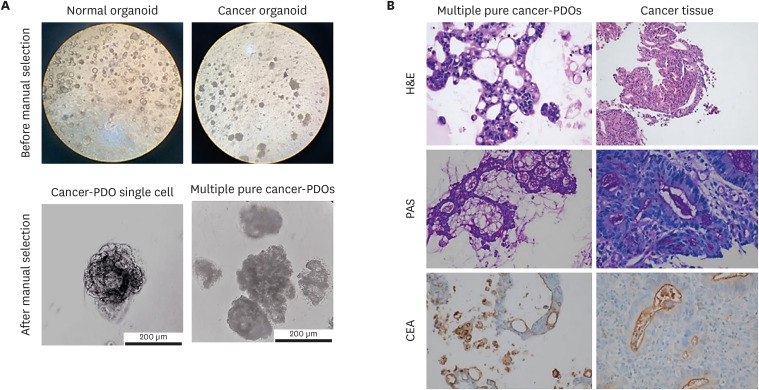 | Fig. 2
Microscopic images of PDOs before and after staining. (A) Unstained PDOs before (top left; generated from normal tissue, top right; generated from cancer tissue, original magnification, ×40) and after manual selection (bottom left; PDO single cell, bottom right; multiple PDOs). (B) Comparative histological and IHC images of the biopsy specimens and pure gastric cancer-PDOs. On H&E stain, histologic features of the gastric cancer-PDO (top left; original magnification, ×400) are similar to those of original tumor tissue from the patient (top right; original magnification, ×100). The intraluminal mucin from gastric cancer-PDO (middle left; original magnification, ×200) and original tumor tissue (middle right; original magnification, ×400) is highlighted by PAS staining. CEA expression as apicoluminal pattern is the same in gastric PDO (bottom left; original magnification, ×200) and original tumor tissue (bottom right; original magnification, ×400).
PDO = patient-derived organoid, IHC = immunohistochemistry, H&E = hematoxylin and eosin, PAS = Periodic acid-Schiff, CEA = carcinoembryonic antigen.

|
As human gastric cancer lesions can have histologic heterogeneity, including normal cells, we further assessed if the gastric cancer-PDOs were truly originated from gastric cancer cells. The tumor from endoscopic biopsy was characterized by tubular formation with atypical tumor cells that was consistent with intestinal type tubular adenocarcinoma (
Fig. 2B, top). IHC staining was additionally performed to show that this was a gastric cancer-PDOs (
Fig. 2B, middle and bottom). Organoids demonstrated mainly tubular structures with loss of polarity and hyperchromasia of tumor cells. PAS staining highlighted the mucin in both biopsy and organoid. IHC studies also revealed the apicoluminal expression of CEA in both specimens. Namely, the comparison of histologic and IHC findings of cancer organoids and the paired biopsy tissues showed that the gastric cancer-PDOs were conveying the characteristics of primary cancer tissues. Based on these results, we successfully demonstrated that cancer-PDOs were generated from gastric cancer tissue.
In this study, we have shown that PDOs can be established from a few small gastric cancer tissues which have been obtained via endoscopic biopsy. Since cancer tissue is composed of multiple clones, the process of isolating organoids into single cells is necessary. We also demonstrated that, despite the heterogenetic cell composition of gastric cancer, single gastric cancer organoids could be manually selected based on the morphology of organoids. Recently, there have been some studies reporting the establishment of gastric cancer organoids using surgically resected specimens of gastric cancer, with further analysis of molecular subtypes, and drug susceptibility tests.
5 This is undoubtedly a very powerful and promising technique to achieve personalized treatment, which is considered an optimal approach to cancer patients. In real clinical practice, however, chemotherapy or molecular targeted therapy is mainly applied to patients with metastatic disease for palliation, who are usually not suitable for surgical treatment. Instead, for these patients with advanced gastric cancer, endoscopic tissue acquisition is a safe, easily applicable, and reasonable modality. Considering this clinical situation, it is essential to confirm whether all these achievements with gastric cancer-PDOs using the surgically obtained tissues, can also be accomplished via endoscopic methods. However, reports on the development of gastric cancer PDOs via endoscopic biopsy method are extremely rare in Korea, not to mention the isolation of single organoids by our modified manual selection method.
9 In our study, organoids were successfully established using a very small amount of gastric cancer tissues obtained via endoscopic biopsy. We obtained 5 pieces of gastric cancer tissues from each patient to produce as large amounts of cancer organoids as possible, and 100 to thousands (50 μL/well) of organoids were generated from one piece of gastric cancer tissue. Previous studies have reported success in establishing drug tests within 3 days by culturing less than 500–1,000 (50 μL/well) of organoids.
7 Accordingly, the organoids derived from small amounts of gastric tissues in our study, would be sufficient in quantity for the purpose of drug screening, which can be practically used in determining the best treatment strategy for advanced gastric cancer in the real clinical practice. To address the major concerns regarding whether these gastric cancer-PDOs could properly represent the characteristics of the original cancer tissues, we have performed histological evaluation and IHC analysis. The results revealed that cancer-PDOs conveyed similar histologic and molecular characteristics to the primary gastric cancer tissue. As this cannot confirm that all the molecular and biologic characteristics perfectly match between the organoid and primary cancer tissue, further investigation of molecular characteristics of the PDOs and primary tumor tissues are needed in future studies.
In conclusion, our study demonstrated the feasibility of endoscopic-derived gastric cancer-PDOs, making it easier to be applied in in-vitro drug sensitivity testing, which will take us one step closer to the precision medicine.
Ethics statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Chung-Ang University (approval No. 1821-007-353). Written informed consent was obtained from all participants.
Go to :







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download