DISCUSSION
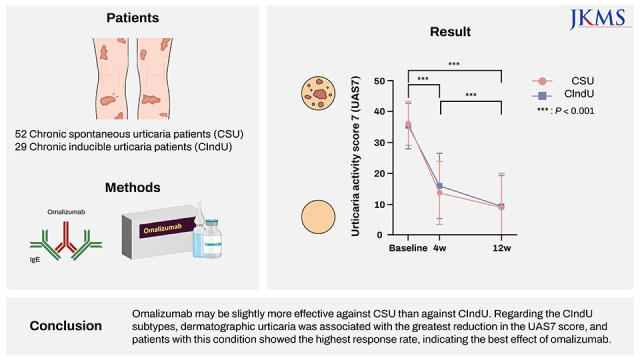
In this study, omalizumab treatment significantly decreased UAS7 in both the CSU and CIndU groups and reduced the numbers of cyclosporine and antihistamine tablets administered. Therefore, we reconfirmed that it was effective against both CSU and CIndU. Improved UAS7 in patients with CSU in this study corresponded to the levels in a meta-analysis of real-world data for CSU, which showed mean 25.6 points of decrease in UAS7.
1 Concerning improvement in CIndU, there have not yet been any studies reporting the difference between baseline and post-treatment UAS7 in patients with CIndU.
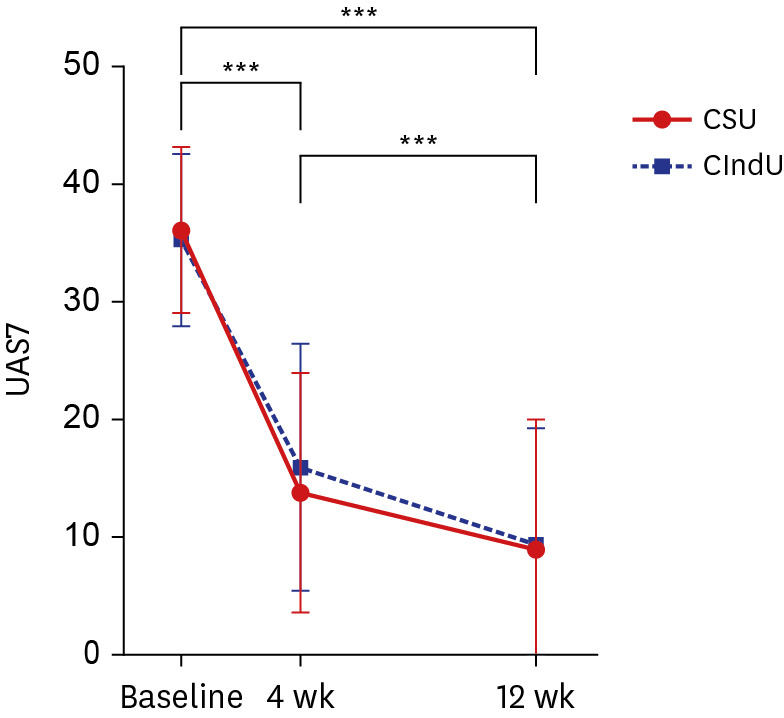
There was no significant difference in UAS7 between the two groups; therefore, it is difficult to say that either is more responsive to treatment. However, the rate of decrease in UAS7 from baseline to week 4 and from baseline to week 12 was larger in the CSU group, indicating a faster decrease in UAS7 in the group (
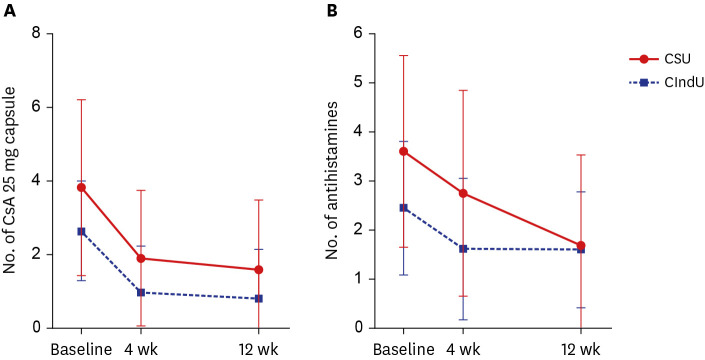
Fig. 1). Furthermore, the number of antihistamine tablets administered every day decreased more significantly in the CSU group, indirectly indicating that CSU symptoms could be controlled better with omalizumab.
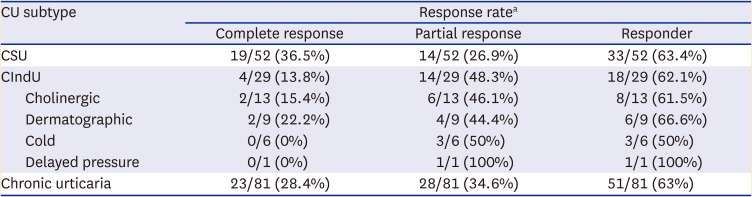
The complete response rate was higher in the CSU group than in the CIndU group (36.5% vs. 13.8%); however, the partial response rate was higher in the latter (48.3% vs. 26.9%). Therefore, the overall response rate was only slightly higher in the CSU group (63.5% vs. 62.1%). The response rates were similar to those in a previous study (week 12: 64.7% [CIndU] and 66.7% [CSU] and increased to 78.6% (CIndU) and 84.6% (CSU) at week 24.
6 These results show that response to omalizumab was slightly better in patients with CSU and continuing omalizumab treatment for 24 weeks may increase response rates in patients who were unresponsive at week 12.
8
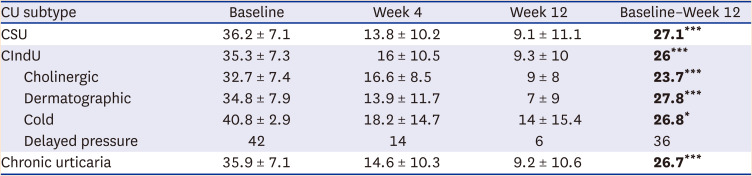
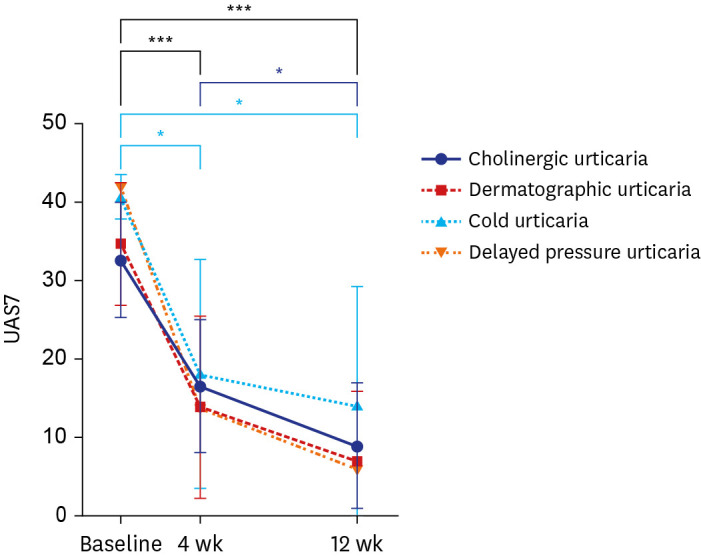
When CIndU subtypes were classified and the response rates were compared, dermatographic urticaria had the highest response (66.7%), and the greatest result for a decrease in UAS7 score from baseline to week 12 was 27.8 (
P < 0.0001). Therefore, dermatographic urticaria showed the best response to omalizumab among the CIndU subtypes. In a randomized placebo-controlled trial of omalizumab in dermatographic urticaria,
11 the response rate in the omalizumab 150 mg administration group was 66%. Hence, our study result was almost consistent with that of the randomized controlled study.
11 Notably, there was no significant difference in efficacy between the 150 and 300 mg treatment groups, which showed a 58% response rate. Therefore, only administration of 150 mg of omalizumab can effectively treat dermatographic urticaria, which was also confirmed in our study.
Cholinergic urticaria also showed a response in 61.5% of patients, and a reduction in UAS7 score from baseline to week 12 showed a good result of 23.7 (P < 0.0001). Furthermore, when comparing the changes from week 4 to week 12, dermatographic urticaria did not show a significant difference in UAS7, while the UAS7 for cholinergic urticaria significantly decreased (P = 0.037). Regarding cold urticaria, 50% of patients showed a response, and the associated UAS7 decreased significantly at weeks 4 (P = 0.036) and 12 (P = 0.017) compared to baseline. In a patient with delayed pressure urticaria, baseline UAS7 of 42 points decreased to 6 points at week 12, confirming omalizumab’s effect.
To date, three studies have compared the effects of omalizumab per CIndU subtypes. The complete response rate was the highest for delayed pressure urticaria in one study.
12 In a retrospective, real-life study,
13 delayed pressure urticaria showed the highest response rate, followed by cold urticaria and cholinergic urticaria. In a study of 80 patients with CIndU,
3 the best response to omalizumab was shown by solar urticaria and a relatively poor response was shown by dermatographic urticaria compared to other subtypes. Since the definitions of response were different in each study and the omalizumab dose administered was differed, it seems difficult to clearly determine which CIndU subtype is the most responsive based on the results obtained hitherto.
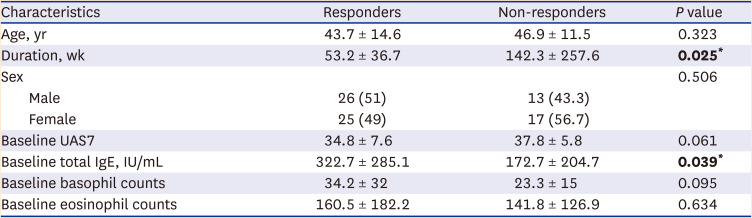
In comparison between responders and non-responders, the duration of chronic urticaria was longer in the non-responders (
P = 0.025). Approximate mean duration of responders was 53.2 weeks, and that of non-responders was 142.3 weeks. Therefore, the duration of chronic urticaria of non-responders was approximately three times longer than that of responders. In contrast, baseline total IgE levels were significantly higher in responders (
P = 0.039); therefore, patients with higher baseline total IgE levels responded better to omalizumab. This finding was consistent with those of previous studies.
1415 Therefore, baseline total IgE levels should be checked in all patients with chronic urticaria in whom omalizumab administration is considered because it can be used to predict treatment response. Additionally, omalizumab can be actively recommended if total IgE levels are high in patients who do not respond well to other treatments.
This study is meaningful in that it offers the largest scale real-world data on omalizumab treatment in Korean patients with chronic urticaria, especially in that it included many patients with CIndU. Especially, it is meaningful in that it confirmed significant improvements in urticaria symptoms among Korean patients administered 150 mg omalizumab rather than 300 mg. In clinical settings, it is often difficult to prescribe 300 mg of omalizumab due to the burden of price. Therefore, it would be appropriate to prescribe 150 mg first and increase the dose to 300 mg if the response is insufficient.
This study has several limitations. First, this was a retrospective study. Secondly, when we count the number of antihistamine tablets administered daily, we did not distinguish antihistamine types. Furthermore, it did not include patients with chronic urticaria who received omalizumab 300 mg. Additionally, a relatively small number of patients with CIndU were included compared to the number of CSU patients. Other CIndU subtypes, such as solar urticaria, heat urticaria, and aquagenic urticaria, were not included. Therefore, a larger-scale prospective study with more patients and various subtypes of CIndU is required.
In summary, omalizumab 150 mg was effective against both CSU and CIndU and showed significantly improved symptoms. Although there was no significant difference in efficacy between the two groups, CSU symptoms tended to improve faster, and the number of antihistamine tablets administered daily decreased more significantly. Further, the reduction in the UAS7 between baseline and week 12 was larger in the CSU group, and the responder rate was slightly higher among CSU patients. Therefore, omalizumab may be slightly more effective against CSU. Among the CIndU subtypes, dermatographic urticaria was associated with the greatest decrease in UAS7, and showed the highest response rate, showing the best effect on omalizumab. In patients with chronic urticaria, the longer the morbidity of the disease, the less likely it would respond to omalizumab. Conversely, the higher the baseline total IgE level, the better the response to omalizumab.
Go to :





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download