Abstract
Objective
This study aimed to investigate the accuracy and precision of continuous, non-invasive blood pressure obtained using the ClearSight system by comparing it with invasive arterial blood pressure, and to assess the hemodynamic changes using invasive methods and the ClearSight system in patients undergoing cesarean section.
Methods
Arterial pressure was measured invasively with an intra-arterial catheter and non-invasively using the ClearSight system during cesarean section in patients with placenta previa or placenta accreta. Blood pressure measurements obtained using these two means were then compared.
Results
Total 1,277 blood pressure measurement pairs were collected from 21 patients. Under Bland-Altman analysis, the ClearSight system demonstrated an acceptable accuracy with a bias and standard deviation of 8.8±13.4 mmHg for systolic blood pressure, −6.3±7.1 mmHg for diastolic blood pressure, and −2.7±8.0 mmHg for median blood pressure. Cardiac index levels were significantly elevated during fetal delivery and 5 minutes after placental removal, and systemic vascular resistance index levels were significantly decreased during fetal delivery and 40 minutes after placental removal.
Post-partum hemorrhage (PPH) is one of the leading causes of maternal death in South Korea [1], and it is important to establish a multidisciplinary treatment beforehand, especially for pregnant women at a high risk of PPH such as placenta previa [2,3]. Invasive arterial blood pressure (BP) monitoring, which provides continuous monitoring as well as access to blood draws, is useful for the management of patients with PPH during cesarean section and helps maintain adequate circulation [4,5]. However, intra-arterial catheterization is invasive and carries the potential risk of complications, such as nerve injury, infection, and thrombosis [6,7]. The ClearSight system (Edwards Lifesciences, Irvine, CA, USA), a non-invasive hemodynamic monitoring device, measures continuous non-invasive BP, stroke volume (SV), SV variance, and cardiac output (CO) based on the volume clamp method. Several studies on non-pregnant populations have shown excellent accuracy and precision between continuous non-invasive BP monitoring and invasive BP monitoring [8,9]. Furthermore, Juri et al. [10] showed that the ClearSight system could reduce and nausea in patients undergoing cesarean section under spinal anesthesia. However, the accuracy and precision of the ClearSight system have not yet been validated in pregnant women at high risk of PPH.
This study aimed 1) to prospectively evaluate the accuracy and precision of continuous non-invasive BP by comparing them with invasive BP and 2) to assess the hemodynamic changes using the ClearSight system in patients undergoing cesarean section.
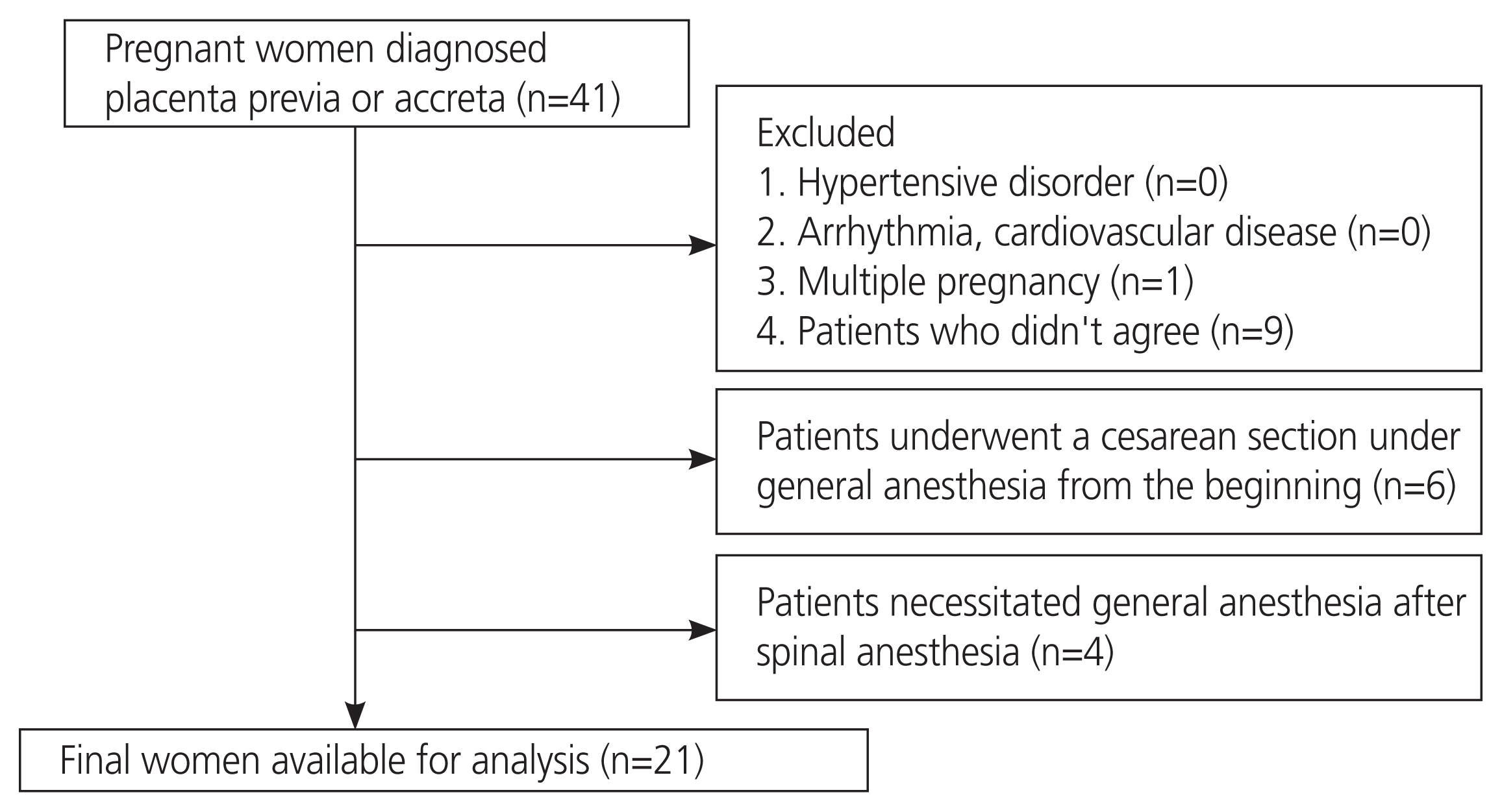
This prospective observational study was approved by the Institutional Review Board (approval number: 4161; October 25, 2018). Written informed consent was obtained from all patients before their inclusion in the study. Patients with placenta previa or placenta accreta were enrolled in the study. Patients with hypertensive disorders during pregnancy, arrhythmias, cardiovascular diseases, and multiple pregnancies were excluded. Patients who underwent cesarean section under general anesthesia were excluded [11]. We also excluded patients who required general anesthesia after spinal anesthesia (Fig. 1). In all cases, we confirmed the difference in systolic blood pressure (SBP) between the right and left arm and, if it was less than 10 mmHg, it was considered to be within the normal range before performing the cesarean section [12].
All patients were allowed to consume clear liquid until 3 hours before surgery and were administered a continuous infusion of Ringer’s lactate solution (200 mL/h) [10]. In the operating room, each patient was positioned on the operating table. Standard hemodynamic monitors, including pulse oximeter and electrocardiography leads were attached. A non-invasive BP cuff (IntelliVue MP70; Philips Electronics, Tokyo, Japan) was attached to the right arm. Each patient rested for 5 minutes while their baseline BP was measured and an intra-arterial catheter was inserted into the left forearm.
To ensure reliable data, the radial artery catheter was flushed, the pressure bag was pressurized and maintained at 300 mmHg, zero-referencing was performed, and the pressure transducer was zeroed at the level of the right atrium and maintained at all times during surgery.
Cesarean section under spinal anesthesia was performed as described below. Patients were administered 0.5% hyperbaric bupivacaine (11.5 mg) and fentanyl (10 μg) in the third lumbar intervertebral space in the right lateral position. After spinal anesthesia, each patient was immediately returned to the supine position, and the sensory block level at T6 was confirmed. From the beginning of the cesarean section, rapid fluid administration with 6% hydroxyethyl starch 130/0.4/9 (Voluven®; Fresenius Kabi, Bad Hamburg, Germany) was started (25 mL/min) until delivery [10]. For patients with an anterior placenta covering the lower uterine wall, we performed the ward technique to avoid transecting the placenta [13,14]. After delivery, the fluid and transfusion management were left to the attending anesthesiologist. Oxytocin infusion was started after placental removal at 100 drops per minutes (5 units of oxytocin per 500 mL serum) to achieve effective uterine contraction, and the on-site hemostatic suturing technique was used to control bleeding from the uterine myometrium [15].
Hemodynamic measurements with the ClearSight system were obtained using a digital cuff of appropriate size after anthropometric configuration by height, weight, sex, and age. The system continuously measures the BP waveform in the finger and calculates the beat-to-beat branchial BP using an algorithm [16–18]. After calibrating the reference transducer to zero, the system was placed on the skin at the heart level. The size of the digital cuff was chosen, and it was placed on the middle finger of the right hand according to the manufacturer’s recommendations. The heart reference system is then zeroed at the midpoint of the right atrium as the reference level. Data for systolic, diastolic, and mean arterial pressures (SBP, diastolic blood pressure [DBP], and mean blood pressure [MBP]), heart rate, and cardiac index (CI) obtained using the ClearSight system were extracted from the EV1000 monitor (Edwards Lifesciences) and registered at 20-second intervals throughout the surgery. Three consecutive data points (obtained over 1 minute) were then aver-aged to yield one datum. The systemic vascular resistance index (SVRI) was calculated assuming a right atrial pressure of 0 mmHg (SVRI=80×MBP/CI) [19]. To ensure simultaneous data analysis, the timing of the data registration was synchronized across that from ClearSight system monitoring. During the cesarean section, hemodynamic measurements were standardized for each woman. Invasive beat-to-beat mean arterial pressures were obtained at intervals of >30 beat and considered to indicate stable and reliable pressure measurements. BP was recorded at 1 minute intervals and stored on an anesthesia monitor (IntelliVue MP70; Philips Electronics Japan Corp., Tokyo, Japan) [20]. Data considered to be artifacts were excluded based on the ClearSight system auto-calibration function and if they were radial artery artifacts or ClearSight system artifacts. Auto-calibration was performed at least once in every 70 heart beats to keep the finger arteries open and of a constant diameter. In addition, auto-calibration was performed when the BP measurement was temporarily interrupted for two or more beats. When auto-calibration was performed, SBP, DBP, and MBP had the same values, which increased gradually. Therefore, it is possible to discriminate such data as artifacts. Radial artery artifacts, which result from blood sampling and flushing, can be discriminated because SBP and DBP have the same values. The ClearSight system artifacts, which occurs owing external pressure on the ClearSight system cuff, can be recognized as extreme outliers.
For the comparison of BP measurements obtained from the intra-arterial catheter and the ClearSight system, bias was defined as the mean difference between the two methods; 95% limits of agreement (LOA) were calculated as bias±(1.96×standard deviation [SD]).
During cesarean section, 12 defined time points for SBP, DBP, MBP, heart rate, and CI were obtained from the ClearSight system. These time points were as follows: 1) before the surgery, 2) at the time of delivery, 3) at the time of placental removal, 4) 5, 5) 10, 6) 15, 7) 20, 8) 25, 9) 30, 10) 40, 11) 50, and 12) 60 minutes after placental removal. Non-invasive measurements of hemodynamic parameters at each of these 12 points were documented, and their medians for each patient were compared.
Continuous variables and categorical variables were expressed as means (ranges) and numbers (%), respectively. To evaluate the accuracy and precision of the ClearSight system for BP measurement, compared to intra-arterial catheter, regression analysis and a Bland-Altman plot with multiple measurements per subject were utilized to compare SBP, DBP, and MBP. Estimations were made of the 95% confidence interval of the bias and the LOA, which were calculated as bias±(1.96×SD) [21]. BP obtained from the ClearSight system was acceptable if precision and accuracy were less than 5 mmHg for bias and 8 mmHg for LOA, based on the standards recommended by the Association for the Advancement of Medical Instrumentation (AAMI) [22]. Statistical analyses were performed using XLSTAT version 2021.2.2 (Addinsoft Inc., New York, NY, USA), bell curve for Excel (Social Survey Research Information Co., Ltd., Tokyo, Japan), and MedCalc statistical software version 20.006 (MedCalc Software Ltd., Ostend, Belgium; 2021).
Of the 41 registered patients, 20 were excluded from the study. Of the 21 cases, 11 (52.4%) were primigravida, eight (38.1%) underwent emergency cesarean section, 18 (85.7%) had placenta previa, and three (14.3%) had placenta accreta. The characteristics of the maternal and neonatal outcomes and perioperative data are shown in Table 1. The median body mass index (BMI) at cesarean section was 25.3 kg/m2, and the median operation time was 61 minutes. The median blood loss was 1,760 mL. Oxytocin was administered to all patients.
A total of 1,277 BP measurement pairs were collected from the 21 cases. The results of the regression analyses of SBP, DBP, and MBP are shown in Fig. 2. The correlation coefficients were 0.712, 0.788, and 0.802 for SBP, DBP, and MBP respectively. The results of the Bland-Altman plot with multiple measurements per subject are shown in Fig. 3. The bias and SD were 8.8±13.4 mmHg for SBP, −6.3±7.1 mmHg for DBP, and −2.7±8.0 mmHg for MBP. The Association for the AAMI controls the standards for BP equipment for measurement in human patients (American National Standards Institute, AAMI; 2008). The AAMI guidelines state that a paired reading must have a mean difference of less than 5 mmHg and a mean SD of less than 8 mmHg. In our study, the Bland-Altman analysis indicated that MBP results measured with the ClearSight system met the AAMI standards; therefore, it was apparent that the ClearSight system produced results that were in good agreement with the MBP measurements.
The variation of MBP obtained from the intra-arterial catheter and the ClearSight system are shown Fig. 4A, B. Compared with the MBP measured before the cesarean section, MBP was significantly decreased after 5 minutes of placental removal and returned to the level before the cesarean section within 50 minutes of placental removal. The variation of hemodynamic parameters obtained from the ClearSight system are shown in Fig. 4C, D. CI shown in Fig. 4C was lowest at the time of preoperation and significantly increased during fetal delivery and after 5 minutes of placental removal (maximum of 17.8% elevation). SVRI was decreased at the time of fetal delivery and lowest after 5 minutes of placental delivery (a 29.5% drop compared with the preoperation level; P<0.001); it continued this lowered level until 40 minutes of placental delivery and increased afterward (Fig. 4D).
In this study, MBP measurements obtained from the Clear-Sight system showed excellent accuracy and precision compared to that of the currently used invasive monitoring system in patients undergoing cesarean section under spinal anesthesia. In addition, using the ClearSight system, we measured the hemodynamic changes during cesarean section non-invasively that showed an elevation in CI during fetal delivery and 5 minutes after placental removal, as well as a decrease in SVRI during fetal delivery and 40 minutes after placental removal, compared with those at the pre-operative period.
Automated digital sphygmomanometers are fairly reliable, safe, and convenient. They are used intra-operatively as a standard and recommended by the American Society of Anesthesiologists to cycle every 5 minutes [7]. Continuous BP monitoring is useful to identify acute fluctuations in hemodynamic status due to anesthesia, surgical stimulation, or hemorrhage, especially for surgeries predicted with a large amount of hemorrhage, for example, placenta previa, placenta accrete, or cases complicated with large myomas. For continuous BP monitoring, an intra-arterial catheter commonly placed in the radial artery is often used to provide accurate hemodynamic monitoring. However, this procedure is invasive and its risk infection or nerve injury is a concern. Therefore, it is less suitable for repeated or long-term use. Therefore, non-invasive methods are useful and safe alternatives for continuous hemodynamic monitoring.
Usually, the AAMI criteria are used for intermittent non-invasive blood pressure monitoring devices. and according to these criteria, two methods can be used alternatively if the bias is less than 5 mmHg with an SD of less than 8 mmHg. In this study of continuous measurements of mean BP during cesarean section, Bland-Altman analysis for the ClearSight system and intra-arterial catheterization showed excellent accuracy and precision, which revealed a mean bias and precision of 2.779±7.299 mmHg and upper and lower LOA of 17.085 and −11.527 mmHg, respectively. Although a large amount of hemorrhage can cause hemodynamic instability, the accuracy and precision of the measurements obtained from the ClearSight system in this study were reliable. We believe that the ClearSight system can be used instead of an intra-arterial catheter to measure BP during cesarean section, even in cases with PPH. We enrolled cases complicated by placenta previa because these cases are predicted to result in massive postpartum hemorrhage, and we can prepare the ClearSight system pre-operatively for such cases. We first showed the reliability of hemodynamic measurements using the ClearSight system in an isologous patient group of relatively young pregnant women without any non-obstetrical complications.
Ueland and Hansen [23] reported maternal cardiovascular dynamics during cesarean section under spinal anesthesia using an indwelling catheter in the branchial artery and superior vena cava. They compared post-delivery cardiovascular dynamics to those of pre-delivery and showed an increased CO by 52 percent and decreased heart rate by an average of 11 beats per minute, while the SV increased by an average of 67 percent. Our result obtained using the ClearSight system seems to be comparable to those obtained using an indwelling catheter in the branchial artery and superior vena cava.
In some previous reports, non-invasive arterial pressure monitoring did not meet the criteria applied to invasive monitoring systems. Stover et al. [24] reported reduced reliability of non-invasive BP measurements obtained from the Nexfin HD, which was the precursor of the ClearSight system. They used the Nexfin HD on 10 critically ill patients who needed cardiovascular monitoring during their stay in the intensive care unit. The mean difference in MBP between the invasive and non-invasive methods was 2±8 mmHg and r2=0.67, although they analyzed only 80 data points for BP. Hohn et al. [25] studied the reliability of the Nexfin HD for BP measurements in 25 critically ill surgical patients, including seven women and 18 men. They analyzed 117 data pairs of invasive and non-invasive measurements of BP, whose bias and SD were 6±12 mmHg. They concluded that non-invasive BP monitoring was not accurate enough to replace intra-arterial invasive BP measurements. However, in their study, the median age of all patients was 63 years (range, 18–82), and the condition of these patients included trauma, hemorrhagic disease, post-operative lung cancer, or sepsis due to pneumonia. In our study, we analyzed only a relatively homologous cohort and excluded patients with diseases likely to affect hemodynamics, such as hypertensive disorders in pregnancy, arrhythmias, cardiovascular diseases, or multiple pregnancies. The discrepancies between our results and those of previous reports might have arisen from population biases.
In this study, the CI or SVRI data obtained from the Clear-Sight system cannot be compared with data calculated from other methods because CI or SVRI measurements usually involve invasive procedures. CI was calculated from CO and body surface area, and SVRI was calculated from MBP and CI. The pulmonary thermodilution method using a pulmonary artery catheter is the gold standard to measure CO [26]. The alternative transpulmonary thermodilution method requires the insertion of a central venous line and an arterial thermistor catheter [27]. Esophageal Doppler can measure CO using the blood flow in the descending aorta via a flexible Doppler probe introduced into the esophagus of anesthetized patients [28]. CO measurement using these methods is reliable, although they require a central vein catheter or a transesophageal probe, both of which involve invasive procedures. The ClearSight system uses a volume clamp method with finger cuffs and relies on photoplethysmography to maintain a constant finger blood volume. Thus, the arterial pressure waveform can be reconstructed, and the CO can be calculated using the CO-trek Algorithm [29]. Several studies have reported the accuracy of CO obtained using the ClearSight system against gold standard techniques, including pulmonary artery catheterization or transesophageal cardiac ultrasonography [30–33]. A new CO monitoring technique is considered to be clinically interchangeable if the squared Pearson correlation coefficient (R2) of the linear regression equation is larger than 0.6 (R>0.77) [34]. These studies showed a good correlation between the ClearSight system and the gold standard methods, resulting in an R of 0.82 to 0.91. Further research is needed to demonstrate the reliability of the CI or SVRI obtained from the ClearSight system in pregnant women.
Our study has several limitations. First, we did not compare the MBP obtained from the ClearSight system with those obtained from sphygmomanometer measurements with an oscillometric cuff. For measurements of BP with an arterial catheter, the damping coefficient depends on several variables, especially the internal radius and length of the catheter itself [35]. Simultaneous BP measurements in the same arm are difficult because neither an arterial catheter nor the ClearSight system can measure BP, whereas a sphygmomanometer cuff tightens around the arm and intercepts arterial blood flow. Second, we did not consider the influence of drugs used during cesarean section. Ephedrine hydrochloride or phenylephrine was used to treat hypotension after spinal anesthesia. Although these drugs can affect hemodynamics, such as CI or SVRI, the timing or dosage of administration was not unified; therefore, it is difficult to exclude the influence of these drugs altogether. Oxytocin was used to prevent atonic bleeding in all cases. The slow injection of oxytocin is associated with a temporary increase in CI, a decrease in SVRI, and no change in BP [36]. In this study, the median usage of oxytocin was 15 units with a range of 10–30 units. The dose and timing of oxytocin use were not unified, and the influence of oxytocin was not considered in our study. Third, this study may lack an adequate sample size to draw a solid conclusion.
In conclusion, we demonstrated the excellent accuracy and precision of the ClearSight system for the measurement of continuous BP, especially MBP, during cesarean section. We believe that the ClearSight system is a useful tool for the management of patients at risk of PPH.
Notes
Ethical approval
This prospective observational study was approved by the Institutional Review Board (approval number: 4161; October 25, 2018).
References
1. Lee KJ, Sohn S, Hong K, Kim J, Kim R, Lee S, et al. Maternal, infant, and perinatal mortality statistics and trends in Korea between 2009 and 2017. Obstet Gynecol Sci. 2020; 63:623–30.

2. Kim HY, Lee D, Kim J, Noh E, Ahn KH, Hong SC, et al. Secular trends in cesarean sections and risk factors in South Korea (2006–2015). Obstet Gynecol Sci. 2020; 63:440–7.

3. Dilla AJ, Waters JH, Yazer MH. Clinical validation of risk stratification criteria for peripartum hemorrhage. Obstet Gynecol. 2013; 122:120–6.

4. Cannesson M, Pestel G, Ricks C, Hoeft A, Perel A. Hemodynamic monitoring and management in patients undergoing high risk surgery: a survey among North American and European anesthesiologists. Crit Care. 2011; 15:R197.

5. Huygh J, Peeters Y, Bernards J, Malbrain ML. Hemodynamic monitoring in the critically ill: an overview of current cardiac output monitoring methods. F1000Res. 2016; 5:F1000 F. aculty Rev-2855.

6. Brzezinski M, Luisetti T, London MJ. Radial artery cannulation: a comprehensive review of recent anatomic and physiologic investigations. Anesth Analg. 2009; 109:1763–81.

7. Bartels K, Esper SA, Thiele RH. Blood pressure monitoring for the anesthesiologist: a practical review. Anesth Analg. 2016; 122:1866–79.
8. Fischer MO, Avram R, Cârjaliu I, Massetti M, Gérard JL, Hanouz JL, et al. Non-invasive continuous arterial pressure and cardiac index monitoring with Nexfin after cardiac surgery. Br J Anaesth. 2012; 109:514–21.

9. Hofhuizen C, Lansdorp B, van der Hoeven JG, Scheffer GJ, Lemson J. Validation of noninvasive pulse contour cardiac output using finger arterial pressure in cardiac surgery patients requiring fluid therapy. J Crit Care. 2014; 29:161–5.

10. Juri T, Suehiro K, Kimura A, Mukai A, Tanaka K, Yamada T, et al. Impact of non-invasive continuous blood pressure monitoring on maternal hypotension during cesarean delivery: a randomized-controlled study. J Anesth. 2018; 32:822–30.

11. Jauniaux E, Alfirevic Z, Bhide AG, Belfort MA, Burton GJ, Collins SL, et al. Placenta praevia and placenta accreta: diagnosis and management: green-top guideline No. 27a. BJOG. 2019; 126:e1–48.
12. Clark CE, Taylor RS, Shore AC, Ukoumunne OC, Campbell JL. Association of a difference in systolic blood pressure between arms with vascular disease and mortality: a systematic review and meta-analysis. Lancet. 2012; 379:905–14.

13. Ward CR. Avoiding an incision through the anterior previa at cesarean delivery. Obstet Gynecol. 2003; 102:552–4.

14. Tahara M, Tachibana D, Hamuro A, Misugi T, Nakano A, Koyama M. The ward technique for anterior placenta previa. Arch Gynecol Obstet. 2021; 303:1375–6.

15. Hamuro A, Tachibana D, Wada N, Kurihara Y, Katayama H, Misugi T, et al. On-site hemostatic suturing technique for uterine bleeding from placenta previa and subsequent pregnancy. Arch Gynecol Obstet. 2015; 292:1181–2.

16. Eeftinck Schattenkerk DW, van Lieshout JJ, van den Meiracker AH, Wesseling KR, Blanc S, Wieling W, et al. Nexfin noninvasive continuous blood pressure validated against riva-rocci/korotkoff. Am J Hypertens. 2009; 22:378–83.

17. Sumiyoshi M, Maeda T, Miyazaki E, Hotta N, Sato H, Hamaguchi E, et al. Accuracy of the ClearSight™ system in patients undergoing abdominal aortic aneurysm surgery. J Anesth. 2019; 33:364–71.

18. Ganzevoort W, Rep A, Bonsel GJ, de Vries JI, Wolf H. Plasma volume and blood pressure regulation in hypertensive pregnancy. J Hypertens. 2004; 22:1235–42.

19. Eyeington CT, Lloyd-Donald P, Chan MJ, Eastwood GM, Young H, Peck L, et al. Non-invasive continuous haemodynamic monitoring and response to intervention in haemodynamically unstable patients during rapid response team review. Resuscitation. 2019; 143:124–33.

20. Heusdens JF, Lof S, Pennekamp CW, Specken-Welleweerd JC, de Borst GJ, van Klei WA, et al. Validation of non-invasive arterial pressure monitoring during carotid endarterectomy. Br J Anaesth. 2016; 117:316–23.

21. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986; 1:307–10.

22. Kim SH, Lilot M, Sidhu KS, Rinehart J, Yu Z, Canales C, et al. Accuracy and precision of continuous noninvasive arterial pressure monitoring compared with invasive arterial pressure: a systematic review and meta-analysis. Anesthesiology. 2014; 120:1080–97.
23. Ueland K, Hansen JM. Maternal cardiovascular dynamics. II. Posture and uterine contractions. Am J Obstet Gynecol. 1969; 103:1–7.
24. Stover JF, Stocker R, Lenherr R, Neff TA, Cottini SR, Zoller B, et al. Noninvasive cardiac output and blood pressure monitoring cannot replace an invasive monitoring system in critically ill patients. BMC Anesthesiol. 2009; 9:6.

25. Hohn A, Defosse JM, Becker S, Steffen C, Wappler F, Sakka SG. Non-invasive continuous arterial pressure monitoring with Nexfin does not sufficiently replace invasive measurements in critically ill patients. Br J Anaesth. 2013; 111:178–84.

26. Seifi A, Elliott RJ, Elsehety MA. Usage of Swan-Ganz catheterization during the past 2 decades in United States. J Crit Care. 2016; 35:213–4.

27. Monnet X, Teboul JL. Transpulmonary thermodilution: advantages and limits. Crit Care. 2017; 21:147.

28. Cholley BP, Singer M. Esophageal Doppler: noninvasive cardiac output monitor. Echocardiography. 2003; 20:763–9.

29. Truijen J, van Lieshout JJ, Wesselink WA, Westerhof BE. Noninvasive continuous hemodynamic monitoring. J Clin Monit Comput. 2012; 26:267–78.

30. Ameloot K, Van De Vijver K, Broch O, Van Regenmortel N, De Laet I, Schoonheydt K, et al. Nexfin noninvasive continuous hemodynamic monitoring: validation against continuous pulse contour and intermittent transpulmonary thermodilution derived cardiac output in critically ill patients. ScientificWorldJournal. 2013; 2013:519080.

31. Broch O, Renner J, Gruenewald M, Meybohm P, Schöttler J, Caliebe A, et al. A comparison of the Nexfin® and transcardiopulmonary thermodilution to estimate cardiac output during coronary artery surgery. Anaesthesia. 2012; 67:377–83.
32. Chen G, Meng L, Alexander B, Tran NP, Kain ZN, Cannesson M. Comparison of noninvasive cardiac output measurements using the Nexfin monitoring device and the esophageal Doppler. J Clin Anesth. 2012; 24:275–83.

33. Bubenek-Turconi SI, Craciun M, Miclea I, Perel A. Non-invasive continuous cardiac output by the Nexfin before and after preload-modifying maneuvers: a comparison with intermittent thermodilution cardiac output. Anesth Analg. 2013; 117:366–72.
34. Critchley LA, Critchley JA. A meta-analysis of studies using bias and precision statistics to compare cardiac output measurement techniques. J Clin Monit Comput. 1999; 15:85–91.
Fig. 2
Regression plot comparing blood pressure measured by the arterial catheter and the ClearSight system for all measurements. (A) Systolic blood pressure (SBP). SBPAC and SBPCS indicate SBP measured by arterial catheter and the ClearSight system, respectively. (B) Diastolic blood pressure (DBP). DBPAC and DBPCS indicate DBP measured by arterial catheter and the ClearSight system, respectively. (C) Mean blood pressure (MBP). MBPAC and MBPCS indicate MBP measured by arterial catheter and the ClearSight system, respectively. SB-PCS, SBP measured by the ClearSight system; CC, correlation coefficient; SBPAC, SBP measured by arterial catheter; DBPCS, DBP measured by the ClearSight system; DBPAC, DBP measured by arterial catheter; MBPCS, MBP measured by the ClearSight system; MBPAC, MBP measured by arterial catheter.
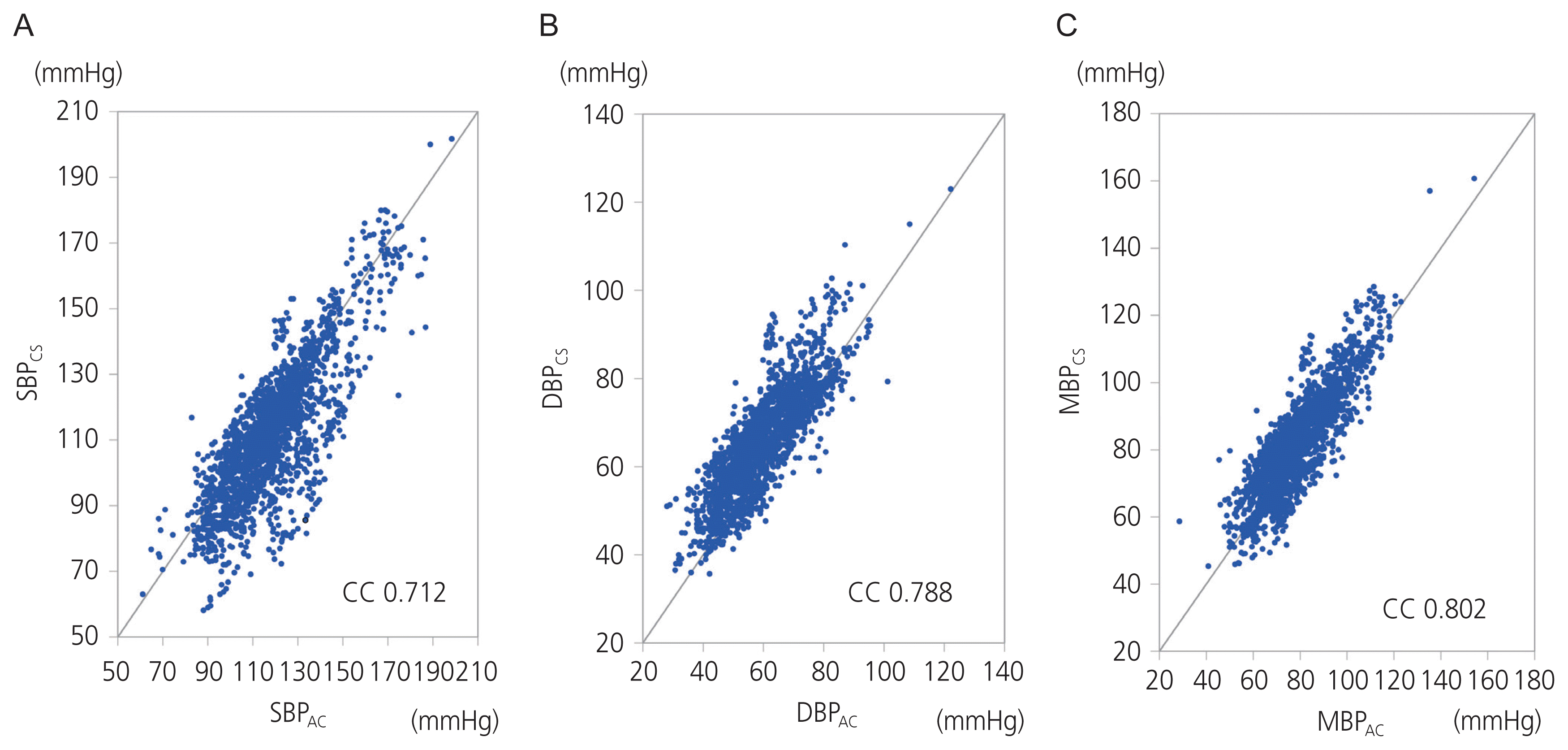
Fig. 3
Bland-Altman analysis evaluating the agreement of blood pressure measured by arterial catheter and the ClearSight system for all measurements. The same symbol (shape and color) indicates the data from the same patient. (A) Systolic blood pressure (SBP). (B) Diastolic blood pressure (DBP). (C) Mean blood pressure (MBP). SBPAC, SBP measured by arterial catheter; SBPCS, SBP measured by the ClearSight system; SD, standard deviation; DBPAC, DBP measured by arterial catheter; DBPCS, DBP measured by the ClearSight system; MBPAC, MBP measured by arterial catheter; MBPCS, MBP measured by the ClearSight system.
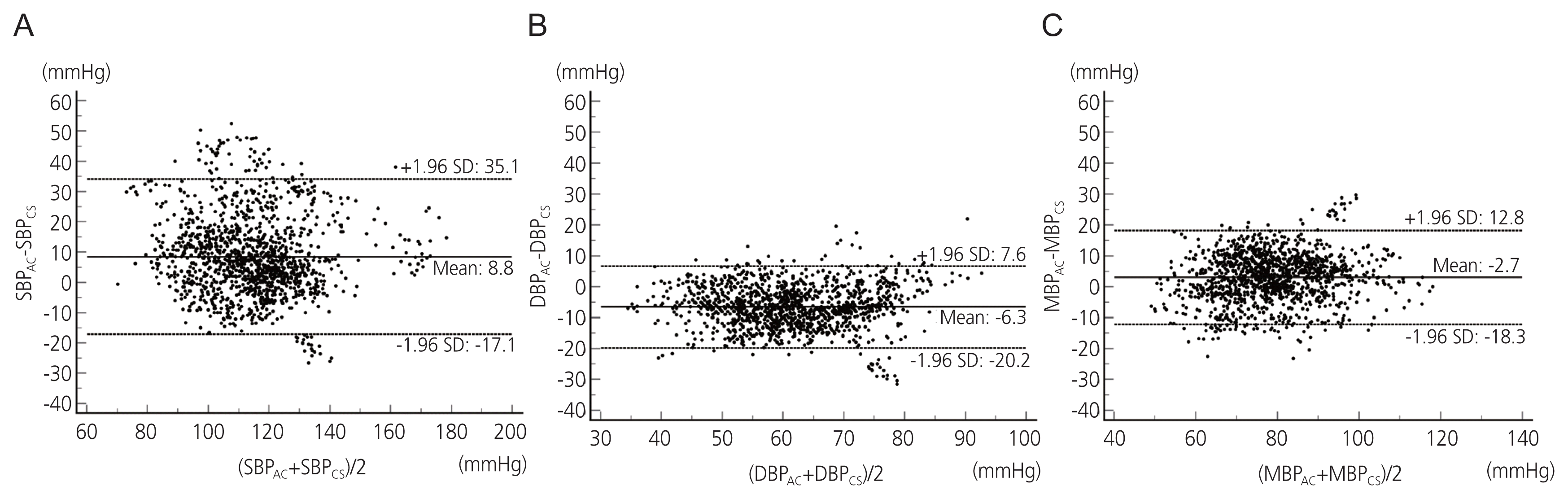
Fig. 4
Transition of representative 12 points of measurement during cesarean section obtained from the ClearSight system. These time points are pre-operative (Pre), at the time of fetal delivery (FD), at the time of placental removal (PR), 5, 10, 15, 20, 25, 30, 40, 50, and 60 minutes after placental removal. We compared each measurement with Pre value. (A) Mean blood pressure measured by arterial catheter. (B) Mean blood pressure measured by the ClearSight system. (C) Cardiac index. (D) Systemic vascular resistance index. m, mintues. *P<0.05.
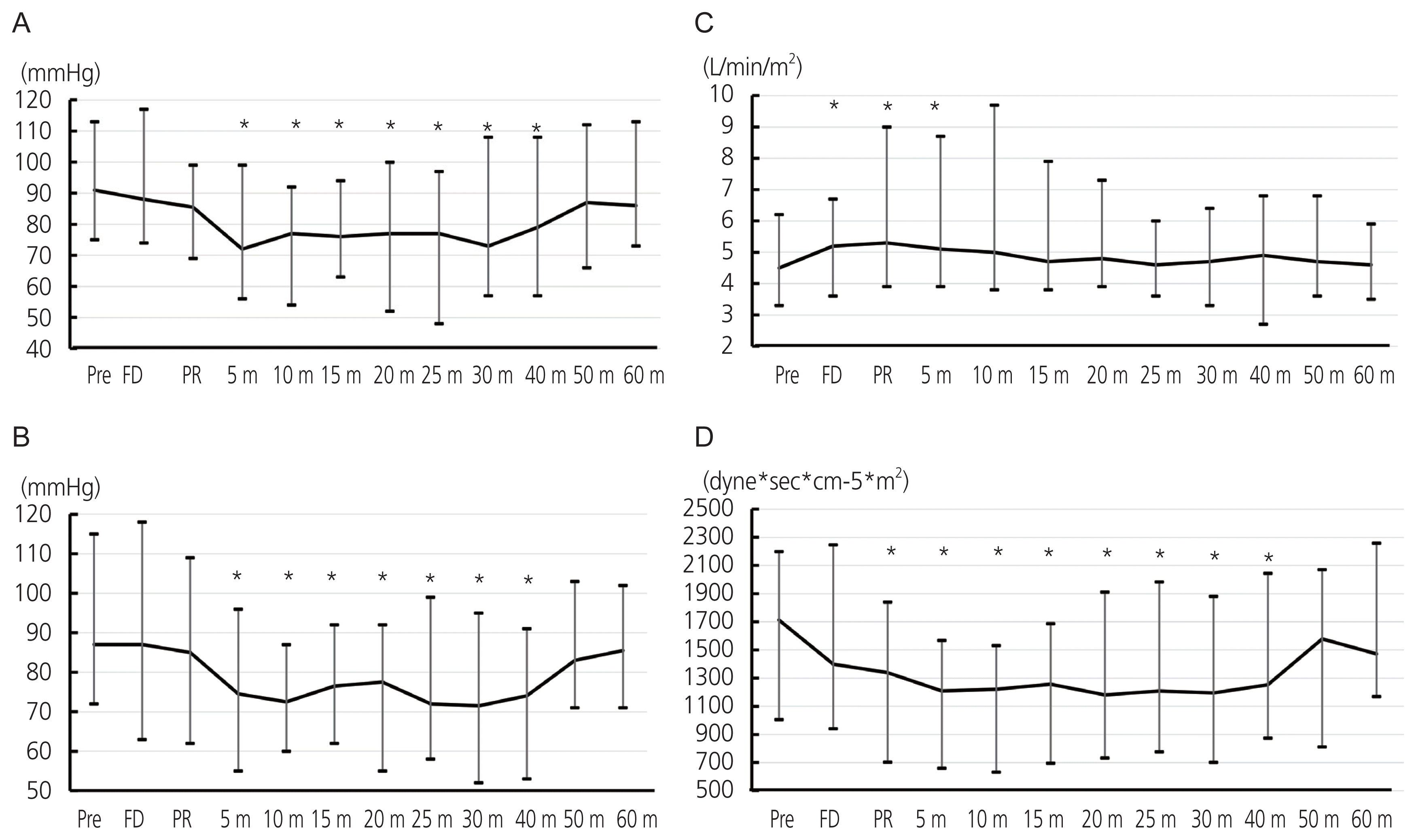
Table 1
Characteristics and perioperative data in 21 case performed cesarean section under spinal aneshtesia




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download