Abstract
Objective
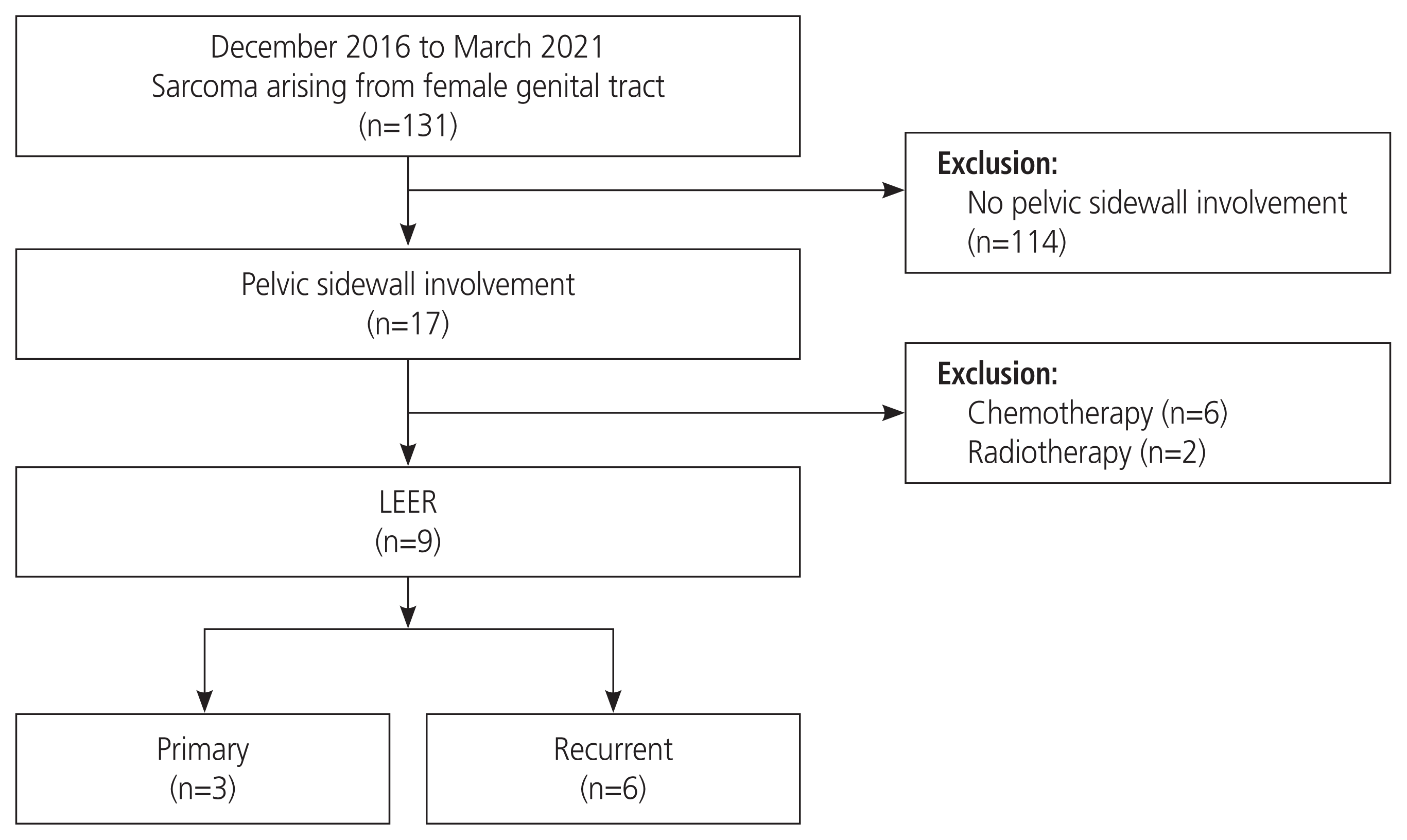
Methods
Results
Acknowledgments
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Jae-Weon Kim has been an Editorial Board of Obstetrics & Gynecology Science; however, he was not involved in the peer reviewer selection, evaluation, or decision process of this article. Otherwise, no other potential conflicts of interest relevant to this article were reported.
References
Table 1
| Characteristic | Value (n=9) |
|---|---|
| Age (yr) | 56 (22–65) |
| Comorbidity | |
| Yesa) | 5 (55.5) |
| None | 4 (44.4) |
| Histology | |
| Carcinosarcoma | 2 (22.2) |
| Leiomyosarcoma | 2 (22.2) |
| Undifferentiated uterine sarcoma | 2 (22.2) |
| Low-grade endometrial stromal sarcoma | 1 (11.1) |
| Mullerian adenosarcoma | 1 (11.1) |
| Synovial sarcoma | 1 (11.1) |
| Disease status at the time of LEER | |
| Primary disease | 3 (33.3) |
| Recurrent disease | 6 (66.7) |
| Initial FIGO stage | |
| I | 4 (44.4) |
| II | 1 (11.1) |
| III | 2 (22.2)b) |
| IV | 2 (22.2) |
| Preoperative radiologic TNM stage | |
| T staging | |
| T2b | 5 (55.6) |
| T4 | 4 (44.4) |
| N staging | |
| N0 | 6 (66.7) |
| N1 | 3 (33.3) |
| M staging | |
| M0 | 7 (77.8) |
| M1 | 2 (22.2) |
| Largest radiologic tumor size prior to LEER (cm) | 10 (2–17.5) |
| Neoadjuvant chemotherapyc) | 2 (22.2)c) |
| Types of prior treatmentd) | |
| Systemic therapy | 5 (55.5) |
| Radiotherapy | 2 (22.2) |
| Surgery+chemotherapy | 4 (44.4) |
| Surgery+radiotherapy | 2 (22.2) |
| Lines of prior chemotherapy | 2 (0–5) |
| Prior systemic treatment regimend),e) | |
| Ifosfamide-combined | 4 (44.4) |
| Doxorubicin only or combined | 3 (33.3) |
| Gemcitabine-docetaxel | 1 (11.1) |
| Paclitaxel-carboplatin | 1 (11.1) |
| Targeted therapy | 1 (11.1) |
| Treatment-free interval before LEER (months) | 3.9 (1.1–38.2) |
| Best response of last treatment before LEER | |
| Complete response | 2 (22.2) |
| Partial response | 2 (22.2) |
| Stable disease | 1 (11.1) |
| Progressive disease | 3 (33.3) |
| Not available | 1 (11.1) |
| Pelvic sidewall tumor locationd) | |
| Infra-iliac acetabulum | 6 (66.7) |
| Infra-iliac ischiopubic | 2 (22.2) |
| Infra-iliac sacrococcygeal | 4 (44.4) |
| Duration of follow-up (months) | 52.7 (11.4–130.4) |
LEER, laterally extended endopelvic resection; FIGO, International Federation of Gynecology and Obstetrics; TNM, The TNM classification of malignant tumors.
a) Comorbidities included hypertension (n=1, 11.1%), diabetes (n=1, 11.1%), dyslipidemia (n=2, 22.2%), thyroid disease (n=1, 11.1%), thromboembolic disease (n=1, 11.1%), and hepatitis (n=1, 11,1%), overlapping conditions included;
Table 2
| Characteristic | Value (n=9) |
|---|---|
| Organ preservation | |
| No | 2 (22.2) |
| Rectum alone | 2 (22.2) |
| Bladder alone | 0 (0.0) |
| Rectum and bladder both | 5 (55.6) |
| Surgical extent | |
| Hysterectomy | 2 (22.2) |
| BSO | 2 (22.2) |
| PLND | 7 (77.8) |
| PALND | 7 (77.8) |
| Cystectomy | 4 (44.4) |
| Vaginectomy | 4 (44.4) |
| Internal iliac vessel resection | 9 (100.0) |
| Pelvic sidewall muscle resection | 8 (88.9) |
| Obturator internus muscle | 6 (66.7) |
| Iliococcygeus muscle | 3 (33.3) |
| Pubococcygeus muscle | 5 (55.6) |
| Coccygeus muscle | 3 (33.3) |
| Ureter ligation and resection | 8 (88.9) |
| Vulvectomy (perineum) | 4 (44.4) |
| Bowel resection | 5 (55.6) |
| Ileal conduit | 4 (44.4) |
| Colostomy | 2 (22.2) |
| Othersa) | 5 (55.5) |
| Pathologic tumor size (cm) | 9.0 (1.8–19.0) |
| Pathologic extent | |
| Uterus | 2 (22.2) |
| Vagina | 4 (44.4) |
| Perineum | 0 (0.0) |
| Bladder and urethra | 7 (77.8) |
| Anus and rectum | 5 (55.6) |
| Pelvic sidewall muscle | 7 (77.8) |
| Internal iliac vessel | 6 (66.7) |
| Tumor grade | |
| Low-grade | 1 (11.1) |
| High-grade | 8 (88.9) |
| Residual tumor | |
| R0 | 8 (88.9) |
| R1 | 1 (11.1) |
| Operation time (minutes) | 300 (135–1,320) |
| Estimated blood loss (mL) | 1,600 (300–22,300) |
| Transfusion | |
| RBC | 3 (0–42) |
| FFP | 0 (0–34) |
| PC | 0 (0–24) |
| Postoperative ICU admission (days) | 1 (0–8) |
| Postoperative complications (according to MSKCC grading system) | |
| Gastrointestinal system (ileus) | |
| Grade 1/2 | 2 (22.2) |
| Grade 3/4 | 0 (0.0) |
| Genitourinary system (urinary incontinence, voiding difficulty) | |
| Grade 1/2 | 2 (22.2) |
| Grade 3/4 | 0 (0.0) |
| Infection | |
| Grade 1/2 | 0 (0.0) |
| Grade 3/4 | 3 (33.3) |
| Nervous system | |
| Grade 1/2 | 4 (44.4) |
| Grade 3/4 | 0 (0.0) |
| Pelvic pain severity | |
| Preoperative NRS | 4 (0–7) |
| Postoperative NRS | 2 (1–3) |
| Preoperative MME (mg/day) | 0 (0–105) |
| Postoperative MME (mg/day) | 0 (0–15) |
| Postoperative adjuvant treatment | |
| No adjuvant treatment | 3 (33.3) |
| Concurrent chemoradiation followed by hormone therapy | 1 (11.1) |
| Chemotherapy | 4 (44.4) |
| Concurrent chemoradiation | 1 (11.1) |
| Treatment response at postoperative 3 months | |
| Complete response | 4 (44.4) |
| Partial response | 0 (0.0) |
| Stable disease | 0 (0.0) |
| Progression or recurrence | 4 (44.4) |
| Not assessable | 1 (11.1) |
| Recurrence | 6 (66.7) |
| Death | 5 (55.6) |
LEER, laterally extended endopelvic resection; BSO, bilateral-salpingo-oophorectomy; PLND, pelvic lymph node dissection; PALND, para-aortic lymph node dissection; RBC, red blood cell; FFP, fresh frozen plasma; PC, platelet concentrate; ICU, intensive care unit; MSKCC, Memorial Sloan Kettering Cancer Center; NRS, numeric rating scale; MME, morphine milligram equivalents.
Table 3
LEER, laterally extended endopelvic resection; FIGO, International Federation of Gynecology and Obstetrics; TNM, the TNM classification of malignant tumors; TFI, treatment free survival; PFS, progression free survival; TRS, treatment-related survival; OS, overall survival; UC, uterine cancer; CCRT, concurrent chemoradiation; CR, complete response; AJCC, American Joint Committee of Cancer; OC, ovarian cancer; PD, progressive disease; FU, follow up; Lt, left; Rt, right; NA, not available.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download