Abstract
The demand for uterine preservation in pelvic reconstructive surgery for uterovaginal prolapse is steadily increasing, and several procedures have been introduced, such as sacrospinous hysteropexy, uterosacral hysteropexy, sacrohysteropexy, and hysteropectopexy. However, the benefits and risks of uterine-preserving surgeries are not well understood. This review discusses the current evidence surrounding uterine-preserving surgery for uterovaginal prolapse repair. This may help surgeons and patients have a balanced discussion on how and on whom to perform uterine-preserving surgery.
Apical support is critical for successful prolapse repair, and performing a concomitant hysterectomy is key when surgeons are planning apical supporting procedures. According to a recent survey, the number of women who favor uterine preservation is steadily increasing [1,2]. Women wish to conserve their uterus for several reasons, including the desire to maintain future fertility and body image, beliefs regarding the adverse effects of sexual dysfunction and their sense of identity, and concerns about the risks of hysterectomy. Survey data indicate that more than one-third of women will choose uterine-preserving prolapse surgery, provided the outcomes are similar, and more than one-fifth of them still prefer uterine preservation, even if it is associated with inferior outcomes [1,2]. Although hysterectomy is routinely performed at the time of uterovaginal prolapse repair, high-level evidence-based data regarding the potential benefits of uterine preservation with hysterectomy are lacking in the literature. Moreover, several reviews have demonstrated that patients may benefit from hysteropexy as opposed to pelvic organ prolapse (POP) surgery with hysterectomy [1,3,4].
Therefore, in this review, we aim to share the current evidence surrounding uterine-preserving surgery in uterovaginal prolapse repair. This may help surgeons and patients have a balanced discussion that will help identify in whom and how to perform uterine-preserving surgery.
To identify suitable patients for uterine-preserving surgery, we first need to know the contraindications for uterine-preserving surgery in patients with uterine prolapse (Table 1) [5–7]. Most studies excluded subjects with an increased risk of uterine cancer. Frick et al. [8] illustrated the need for hysterectomy in women with postmenopausal bleeding, even in those with a negative work-up, because of the increased risk (13%) of unanticipated endometrial pathology. However, none of the premenopausal women with uterovaginal prolapse and normal bleeding patterns or women with a negative evaluation for abnormal uterine bleeding had premalignant or malignant pathology. Among postmenopausal women without bleeding, only 2.6% were diagnosed with endometrial pathologies. This implies that hysterectomy as a preventive measure is not recommended for women at average risk.
Women with cervical elongation or advanced uterine prolapse might not be suitable for hysteropexy, especially vaginal hysteropexy, because the available data show a high prolapse recurrence rate in this group [9,10].
Table 2 lists the advantages and disadvantages of uterine preservation at the time of prolapse surgery [11]. Other than the advantages of maintaining fertility and body image, uterine-preserving surgery has shown superior safety profile in the literature. It has the advantages of a shorter operation time and hospital stay and less blood loss [12,13]. In addition to superior perioperative outcomes, mesh exposure has been consistently found to be lower in sacrohysteropexy (SHP) than in sacrocolpopexy (SCP). A meta-analysis demonstrated that hysterectomy and SCP were associated with a fivefold higher rate of mesh exposure compared to SHP, given the comparable anatomical outcomes [3]. Similarly, hysterectomy at the time of SCP was associated with a fourfold higher risk of mesh exposure, compared to SCP without hysterectomy [3].
One of the disadvantages of hysterectomy is the potential risk of pelvic neuropathy associated with pelvic floor dissection and disruption of the uterosacral cardinal ligament complex, which may further weaken pelvic floor support [14]. In other words, when patients undergo uterine-preserving surgery, they are free from these risks.
The disadvantages of uterine-preserving surgery are as follows: 1) the patient should continue to monitor the cervix and endometrium owing to the small, but present, ongoing risk for cervical or endometrial cancer [11]. 2) Hysteropexy and subsequent hysterectomy after the index surgery are more complex and difficult for the surgeon.
Surgical outcomes are also important aspects to consider when making surgical decisions. Nonetheless, it is common knowledge that uterus-preserving surgery increases the risk of prolapse recurrence, and there are limited data to support this. A recent meta-analysis did not demonstrate that hysterectomy was significantly associated with a lower rate of apical prolapse recurrence (risk ratio, 1.65; 95% confidence interval, 0.88–3.10, P=0.12) [12]. However, the authors mentioned that they found a tendency for hysterectomy to be associated with a lower recurrence rate of apical prolapse in the majority of included studies. Regarding the reoperation rate, the rate of recurrence of apical prolapse was not lower in the hysterectomy group compared with the uterus-preserving group, but the rate of reoperation for POP was lower in the hysterectomy group. It should be acknowledged that the definition of recurrence concerned apical prolapse and the definition of reoperation concerned any vaginal compartment. In addition to the studies included in this meta-analysis, the largest randomized trial on this issue found that sacrospinous hysteropexy was not inferior to vaginal hysterectomy and uterosacral ligament suspension for recurrent apical prolapse at the 12-month follow-up [15]. However, this result should be interpreted with caution due to the inclusion of a large number of women with mild uterine prolapse. Indeed, apical loss beyond the hymen (i.e., POP Quantification point C >0) has been reported as a significant risk factor for surgical failure after vaginal hysteropexy [9,10]. In contrast, Dallas et al. [16] revealed that hysterectomy at the time of prolapse surgery reduced the risk of reoperation for prolapse recurrence at a median follow-up of 4 years. Their report was based on a large population-based study using data from nearly 10,000 women. Unfortunately, the majority of results in the literature have been drawn under a relatively short follow-up period and lack of appropriate control groups [11]. We should pay attention when interpreting all of the above findings since statistically insignificant or conflicting ones can also be clinically relevant [12].
The adverse impact of uterine-preserving surgery on sexual function is uncertain. The only study on this issue demonstrated no difference in the impact on sexual function between women who underwent sacrospinous hysteropexy and women who underwent vaginal hysterectomy [17]. Additionally, studies on hysterectomy for benign conditions have generally found no adverse impact of hysterectomy on sexual function [18–20].
Sacrospinous hysteropexy is the most commonly performed procedure for vaginal hysteropexy using native tissues (Table 3). Usually, the right-sided sacrospinous ligament is identified through extraperitoneal dissection and then attached to the posterior cervix (Fig. 1). Many outcome data have been stacked, and most studies related to vaginal hysteropexy have included sacrospinous hysteropexy as one of their arms [22]. The majority of studies have revealed that sacrospinous hysteropexy is comparable to vaginal hysterectomy and prolapse repair in efficacy, with superior perioperative outcomes [3,11].
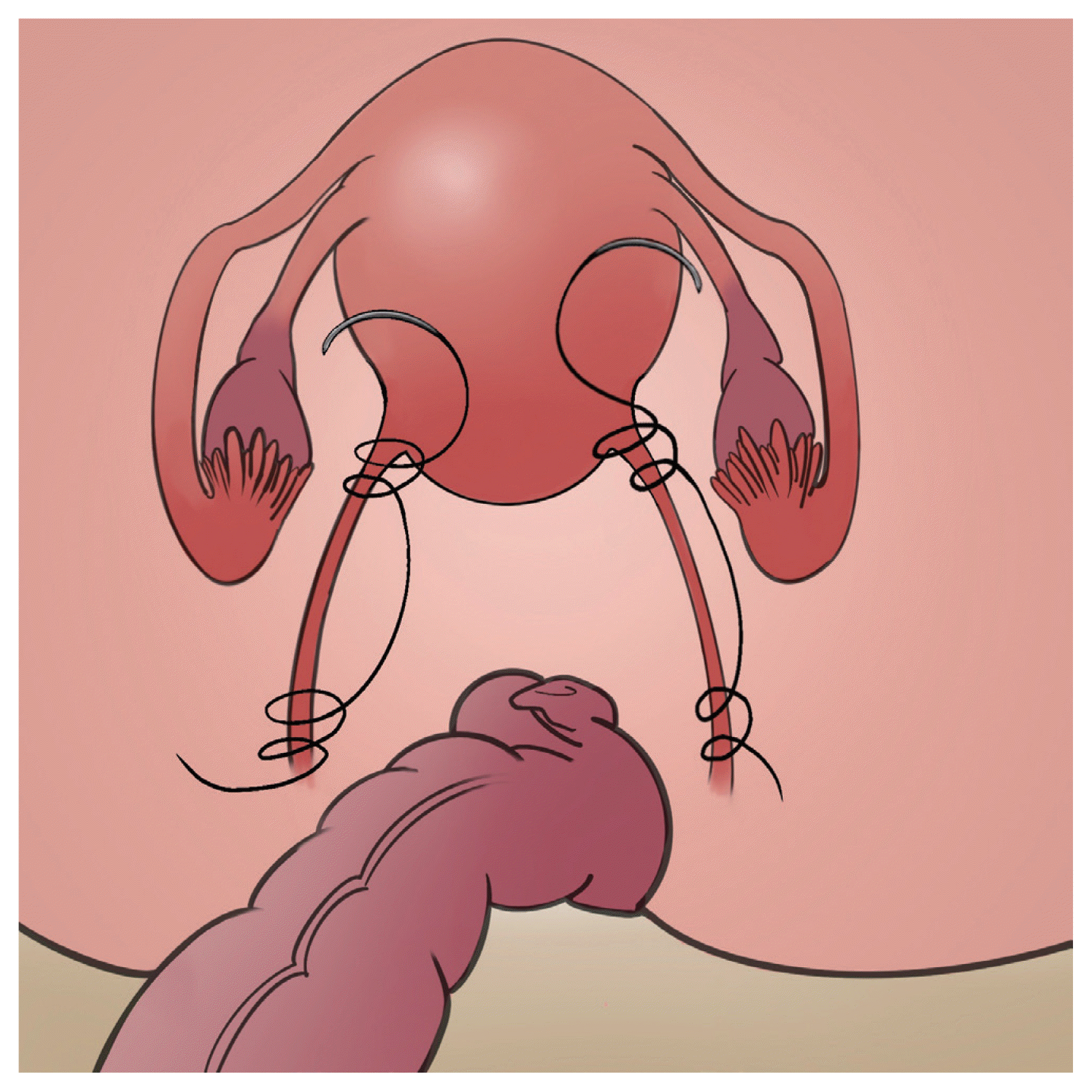
Another technique for hysteropexy using native tissues is uterosacral hysteropexy. It can be performed transvaginally or transabdominally (Table 3). Basically, 1–3 delayed absorbable or nonabsorbable sutures are placed through each uterosacral ligament at or above the ischial spine, and then they are secured to the cervix and upper vagina (Fig. 2). Few studies have reported on the efficacy of transvaginal uterosacral hysteropexy. One study by Romanzi and Tyagi [23] demonstrated no differences in apex durability at 2 years between the transvaginal uterosacral hysteropexy group and the vaginal hysterectomy with uterosacral ligament colpopexy group (96% vs. 96.8%, P=0.90). One systematic review presented a mean objective success rate of 83% (148 of 176 women) after laparoscopic uterosacral hysteropexy compared to 78% (21 of 27 women) after laparoscopic hysterectomy and 88% (22 of 25 women) after vaginal hysterectomy and uterosacral ligament suspension [3]. Owing to the significant variation in the definition of success and surgical technique, a meta-analysis could not be performed for this issue [3]. Although the evidence is limited, transabdominal uterosacral hysteropexy seems to be effective [11].
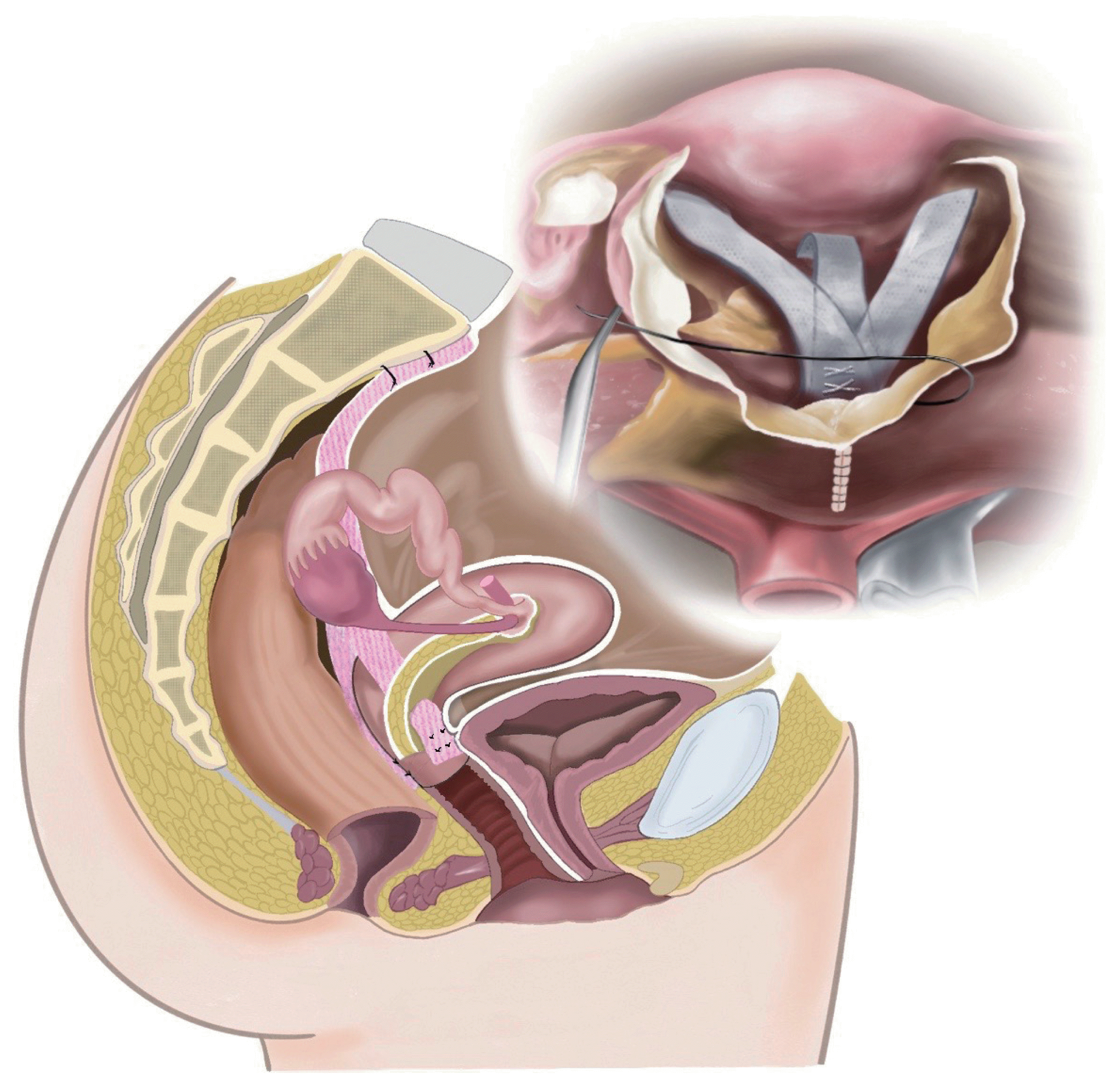
Although there are variations in the approach route and surgical technique (Fig. 3), including the type of mesh and mesh attachment site, evidence regarding SHP is relatively robust compared to that for other uterus-preserving procedures. Many studies have shown that SHP has high and comparable anatomical success rates compared to hysterectomy and SCP, reaching 91% and 92%, respectively (P=0.17) [3,11]. SHP appears to be a safe procedure, similar to SCP, and is even safer with regard to mesh exposure rate. Most studies have shown a higher incidence of mesh exposure when the uterus was removed concomitantly, with a tendency for a lower incidence when the uterus was preserved. For instance, the mesh exposure rate was 3% [24], which was lower than the rates of hysterectomy and SCP (23%) [25]. Hysteropexy techniques that do not place a foreign body near the colpotomy site are thought to be the reason for this [11].
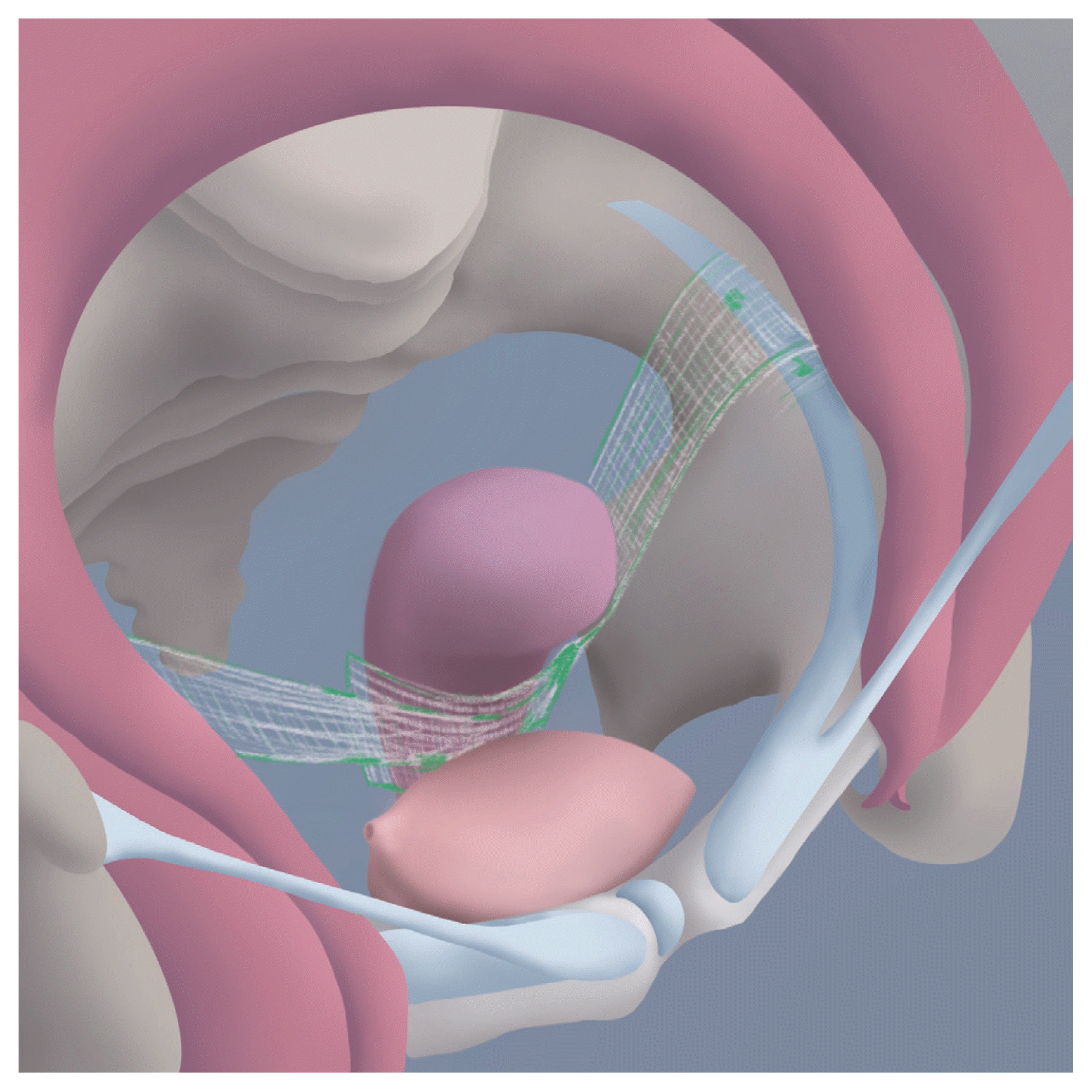
This new technique for prolapse surgery was described in 2010 [26]. This is a transabdominal surgery that uses a mesh (Table 3). As its name indicates, the bilateral iliopectineal ligaments (Cooper’s ligaments) are the anchoring ends for this procedure. Soft tissue between the round ligament and external iliac vein is pushed downward with blunt dissection up to the region near the obturator nerve; therefore, approximately 4–5 cm of the iliopectineal ligament is exposed. For patients who want to preserve their uterus, the lower anterior segment of the uterus is prepared by dissecting the anterior peritoneum of the uterus. The center of the mesh is fixed to the anterior uterine wall and the ends of the mesh are fixed to the iliopectineal ligaments (Fig. 4). Finally, the peritoneum above the mesh is closed with absorbable suture material to avoid intrapelvic adhesions [26,27]. Although adequate data are lacking, especially for hysteropectopexy, several case series have shown promising results [27,28]. These studies reported no recurrence of prolapse during short-term (10–14 months) follow-up, with a low complication rate.
While exploring the literature for hysteropexy, we encountered many studies on vaginal mesh hysteropexy. Various mesh kits that can easily accommodate uterine preservation and correct vaginal prolapse have been developed and have become popular. However, most transvaginal mesh products have been withdrawn from the market since the 2011 U.S. Food and Drug Administration’s (FDA) announcement that identified serious safety and efficacy concerns for transvaginal mesh [29]. In 2019, the FDA ordered companies producing mesh products indicated for the transvaginal repair of POP to stop selling and distributing their products in the United States [30]. A Cochrane systematic review of seven randomized trials that compared native tissue repair with synthetic mesh vaginal prolapse repair found that more women in the mesh group required reoperation for the combined outcomes of prolapse, stress incontinence, or mesh complications [31]. The rate of mesh exposure was 12%, and 8% of women required reoperation for mesh exposure up to 3 years.
Seventeen pregnancies from several different studies were reported after sacrospinous hysteropexy, with half of the deliveries being vaginal and half being cesarean deliveries at term, followed by 2 recurrences [3]. Several case reports have noted pregnancies and deliveries after uterosacral hysteropexy as well as SHP, with very few prolapse recurrences [11]. Despite relatively good outcomes, the available evidence is limited and of low quality. Thus, it is unclear how hysteropexy affects fertility and pregnancy, or how pregnancy affects hysteropexy.
Basically, conservative management, such as observation, pessary, or pelvic floor muscle exercise, is the first line of treatment for women with uterovaginal prolapse who have not completed childbearing. Hysteropexy may be an option for women for whom a pessary cannot be fitted or for younger patients who refuse conservative management for a prolonged time [3]. Some experts recommended vaginal hysteropexy using native tissue for this group of women, while they perform SHP using only a posterior mesh for those with stage IV prolapse. They assume that placing an anterior mesh could restrict lower anterior uterine segment changes, which are required for uterine expansion [11].
In summary, hysteropexy may be a considerable alternative for women with mild uterine prolapse who desire to undergo the procedure, have no contraindications to uterine preservation, and understand the prognosis of uterine-preserving surgery [21]. Vaginal sacrospinous hysteropexy, vaginal or abdominal uterosacral hysteropexy, SHP, and hysteropectopexy are the available options.
Acknowledgments
Presented at the 107th Annual Congress of Korean Society of Obstetrics and Gynecology, October 1–2, 2021.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Myung Jae Jeon has been an Editorial Board of Obstetrics & Gynecology Science; however, he was not involved in the peer reviewer selection, evaluation, or decision process of this article. Otherwise, no other potential conflicts of interest relevant to this article were reported.
References
1. Frick AC, Barber MD, Paraiso MF, Ridgeway B, Jelovsek JE, Walters MD. Attitudes toward hysterectomy in women undergoing evaluation for uterovaginal prolapse. Female Pelvic Med Reconstr Surg. 2013; 19:103–9.

2. Korbly NB, Kassis NC, Good MM, Richardson ML, Book NM, Yip S, et al. Patient preferences for uterine preservation and hysterectomy in women with pelvic organ prolapse. Am J Obstet Gynecol. 2013; 209:470e1–6.
4. Ridgeway B, Frick AC, Walter MD. Hysteropexy. A review. Minerva Ginecol. 2008; 60:509–28.
5. Gutman RE. Does the uterus need to be removed to correct uterovaginal prolapse? Curr Opin Obstet Gynecol. 2016; 28:435–40.

6. Kow N, Goldman HB, Ridgeway B. Management options for women with uterine prolapse interested in uterine preservation. Curr Urol Rep. 2013; 14:395–402.

7. Kim BH, Lee SB, Na ED, Kim HC. Correlation between obesity and pelvic organ prolapse in Korean women. Obstet Gynecol Sci. 2020; 63:719–25.

8. Frick AC, Walters MD, Larkin KS, Barber MD. Risk of unanticipated abnormal gynecologic pathology at the time of hysterectomy for uterovaginal prolapse. Am J Obstet Gynecol. 2010; 202:507e1–4.

9. Suh DH, Jeon MJ. Risk factors for the failure of iliococcygeus suspension for uterine prolapse. Eur J Obstet Gynecol Reprod Biol. 2018; 225:210–3.

10. Lin TY, Su TH, Wang YL, Lee MY, Hsieh CH, Wang KG, et al. Risk factors for failure of transvaginal sacrospinous uterine suspension in the treatment of uterovaginal prolapse. J Formos Med Assoc. 2005; 104:249–53.
11. Ridgeway BM. Does prolapse equal hysterectomy? The role of uterine conservation in women with uterovaginal prolapse. Am J Obstet Gynecol. 2015; 213:802–809.

12. de Oliveira SA, Fonseca MCM, Bortolini MAT, Girão MJBC, Roque MT, Castro RA. Hysteropreservation versus hysterectomy in the surgical treatment of uterine prolapse: systematic review and meta-analysis. Int Urogynecol J. 2017; 28:1617–30.

13. Meriwether KV, Balk EM, Antosh DD, Olivera CK, Kim-Fine S, Murphy M, et al. Uterine-preserving surgeries for the repair of pelvic organ prolapse: a systematic review with meta-analysis and clinical practice guidelines. Int Urogynecol J. 2019; 30:505–22.

14. Nesbitt RE Jr. Uterine preservation in the surgical management of genuine stress urinary incontinence associated with uterovaginal prolapse. Surg Gynecol Obstet. 1989; 168:143–7.
15. Detollenaere RJ, den Boon J, Stekelenburg J, IntHout J, Vierhout ME, Kluivers KB, et al. Sacrospinous hysteropexy versus vaginal hysterectomy with suspension of the uterosacral ligaments in women with uterine prolapse stage 2 or higher: multicentre randomised non-inferiority trial. BMJ. 2015; 351:h3717.

16. Dallas K, Elliott CS, Syan R, Sohlberg E, Enemchukwu E, Rogo-Gupta L. Association between concomitant hysterectomy and repeat surgery for pelvic organ prolapse repair in a cohort of nearly 100,000 women. Obstet Gynecol. 2018; 132:1328–36.

17. Jeng CJ, Yang YC, Tzeng CR, Shen J, Wang LR. Sexual functioning after vaginal hysterectomy or transvaginal sacrospinous uterine suspension for uterine prolapse: a comparison. J Reprod Med. 2005; 50:669–74.
18. Yazbeck C. Sexual function following hysterectomy. Gynecol Obstet Fertil. 2004; 32:49–54.
19. Mokate T, Wright C, Mander T. Hysterectomy and sexual function. J Br Menopause Soc. 2006; 12:153–7.

20. Danesh M, Hamzehgardeshi Z, Moosazadeh M, Shabani-Asrami F. The effect of hysterectomy on women’s sexual function: a narrative review. Med Arch. 2015; 69:387–92.

21. Jeon MJ. Surgical decision making for symptomatic pelvic organ prolapse: evidence-based approach. Obstet Gynecol Sci. 2019; 62:307–12.

22. Ridgeway BM, Frick AC. Uterine conservation for the surgical treatment of uterovaginal prolapse. Walter Mark, Karram M, editors. Urogynecology and reconstructive pelvic surgery. Phildelphia (PA): Elsevier Saunders;2015. p. 383–99.
23. Romanzi LJ, Tyagi R. Hysteropexy compared to hysterectomy for uterine prolapse surgery: does durability differ? Int Urogynecol J. 2012; 23:625–31.

24. Barranger E, Fritel X, Pigne A. Abdominal sacrohysteropexy in young women with uterovaginal prolapse: long-term follow-up. Am J Obstet Gynecol. 2003; 189:1245–50.

25. Tan-Kim J, Menefee SA, Luber KM, Nager CW, Lukacz ES. Prevalence and risk factors for mesh erosion after laparoscopic-assisted sacrocolpopexy. Int Urogynecol J. 2011; 22:205–12.

26. Banerjee C, Noé KG. Laparoscopic pectopexy: a new technique of prolapse surgery for obese patients. Arch Gynecol Obstet. 2011; 284:631–5.

27. Yu EH, Jung HE, Noh HK, Joo JK. Initial experience of laparoscopic pectopexy for apical prolapse in South Korea. J Menopausal Med. 2020; 26:165–8.

28. Tahaoglu AE, Bakir MS, Peker N, Bagli İ, Tayyar AT. Modified laparoscopic pectopexy: short-term follow-up and its effects on sexual function and quality of life. Int Urogynecol J. 2018; 29:1155–60.

29. Food and Drug Administration (FDA). Urogynecologic surgical mesh: update on the safety and effectiveness of transvaginal placement for pelvic organ prolapse. Silver Spring (MD): FDA;2011.
30. Food US; Drug Administration (FDA). FDA takes action to protect women’s health, orders manufacturers of surgical mesh intended for transvaginal repair of pelvic organ prolapse to stop selling all devices. Silver Spring (MD): FDA;2019.
Fig. 1
Native tissue sacrospinous hysteropexy with the posterior cervix attached to the right sacrospinous ligament.
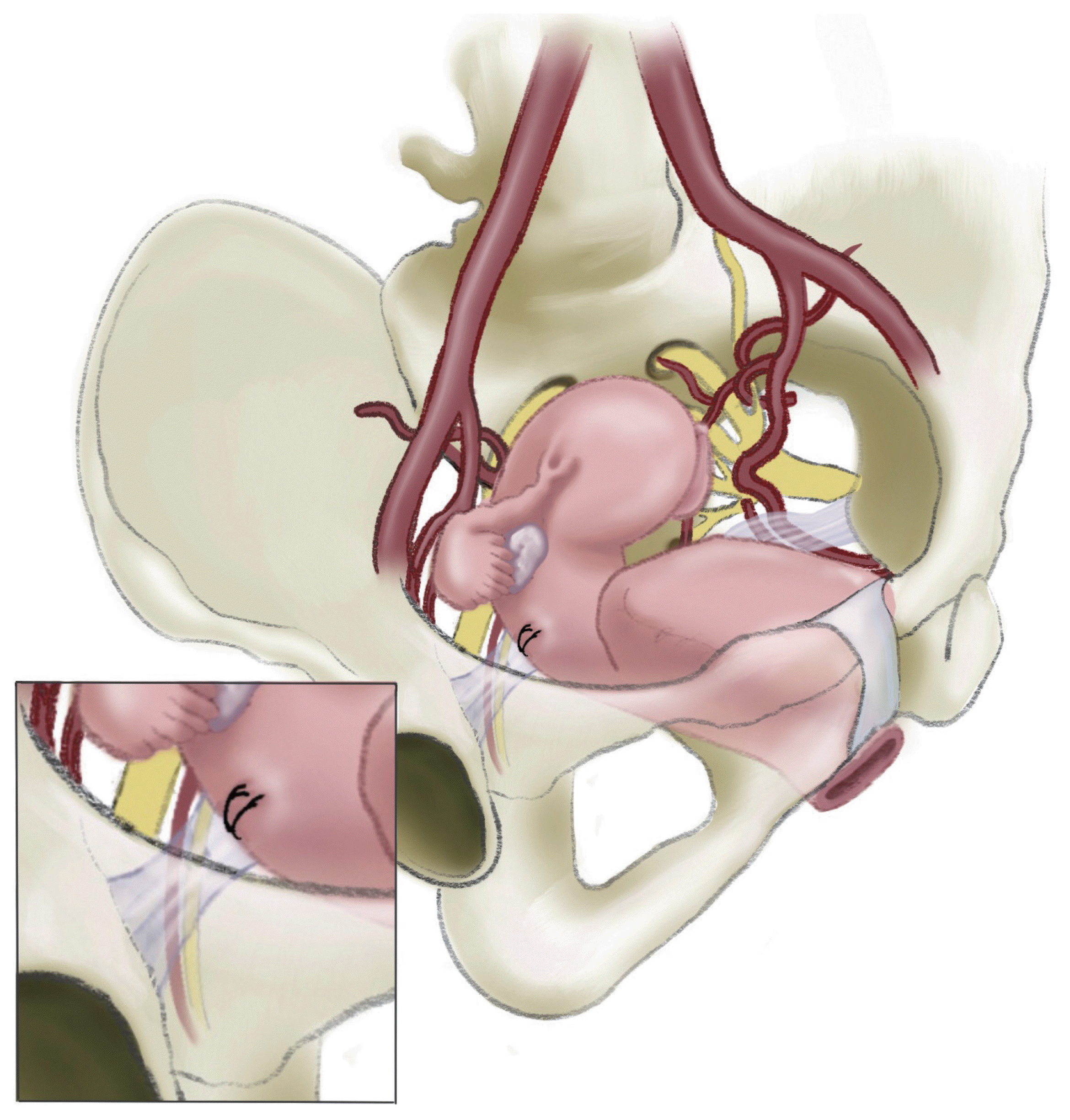
Table 1
Contraindications for uterine preservation
Table 2
Advantages and disadvantages of uterine preservation at the time of prolapse surgery
Table 3
Procedures for correcting uterine prolapse with uterine preservation




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download