This article has been
cited by other articles in ScienceCentral.
ABSTRACT
Cardiac rhabdomyomas are typically presented in the tuberous sclerosis. Although benign and often associated with spontaneous regression, in rare circumstances huge mass size and critical location can lead to heart failure, ventricular outflow tract obstruction and refractory tachyarrhythmias. An 1-day-old girl was diagnosed as cardiac tumor during perinatal period. At birth, transthoracic echocardiography revealed huge cardiac mass located in septal area of both ventricle measuring 34×30 mm. It protruded into the left ventricular (LV) outflow tract, potentially obstructing it. A surface ECG revealed atrial tachycardia with nonsustained ventricular tachycardia with heart rate of 217 beats per min. The tachyarrhythmias were controlled with intravenous amiodarone. Reduction of the giant cardiac mass was treated with mammalian target of rapamycin pathway inhibitor sirolimus. However, she unfortunately died at 10 days-old because of sudden cardiac arrest maybe due to LV outflow tract obstruction during therapy. Gene analysis revealed TSC2 mutation after death. (Ewha Med J 2022;45(3):e5)
Keywords: Rhabdomyoma, Tachycardia, Ectopic atrial, Tachycardia, ventricular, Tuberous sclerosis
Introduction
Rhabdomyomas are the most common cardiac tumor in pediatric patients. In many cases, they are associated with tuberous sclerosis which is an autosomal dominant disorder caused by mutations in the TSC1 or TSC2 genes that are part of mammalian target of rapamycin (m-TOR) pathway [
1]. Although benign and often associated with spontaneous regression, in rare circumstances huge mass size and critical location can lead to heart failure, ventricular outflow tract obstruction and refractory tachyarrhythmias [
2]. Here we present a fatal case of mixed atrial and ventricular tachycardia (VT) in a newborn with giant cardiac rhabdomyoma and tuberous sclerosis.
Case Presentation
An 1-day-old girl was born by cesarian section at a gestational age of 36 weeks with birth weight of 2,710 g. Cardiac tumor was diagnosed during perinatal period. At birth, transthoracic echocardiography revealed huge cardiac mass located in septal side of both ventricle involving free wall of the ventricle and the outflow tract measuring 34×30 mm in its longest axis. It protruded into the left ventricular (LV) outflow tract, potentially obstructing it (
Fig. 1). And other small multiple cardiac masses were located in papillary muscle and chordae tendinae of LV. No other visible mass was observed in the atria, and ventricular systolic function was preserved. Brain ultrasound showed multiple cortical and subcortical tubers on both fronto-parieto-occipital area related to the tuberous sclerosis (
Fig. 2). A 12-lead surface ECG revealed atrial tachycardia (AT) with nonsustained VT which showed wide QRS tachycardia with heart rate of 217 beats per min (
Fig. 3A). To reduction of the giant cardiac mass, m-TOR inhibitor sirolimus was treated. In addition, the tachyarrhythmias were controlled to intravenous amiodarone, which was given 5 mg/kg bolus followed by continuous infusion up to 10 mcg/kg/min. After that, atrial tachyarrhythmias were converted to nonsustained AT and then frequent atrial premature beats with bigeminy (
Fig. 3B,
Fig. 3C). No VT was observed any more. However, she unfortunately died at 10 days-old because of sudden cardiac arrest maybe due to LV outflow tract obstruction during therapy. Gene analysis revealed TSC2 mutation after death.
Fig. 1.
Transthoracic echocardiography of rhabdomyoma. (A) Huge cardiac mass (asterisks) located in both ventricle measuring 34×30 mm on apical four chamber view. Small multiple cardiac masses (arrows) were located in papillary muscle and chordae tendinae of LV. (B) Cardiac mass (asterisks) protruded into the left ventricular (LV) outflow tract, potentially LV outflow tract obstruction on parasternal long axis view. RV, right ventricle; RA, right atrium; LA, left atrium; LV, left ventricle; AAo, ascending aorta.
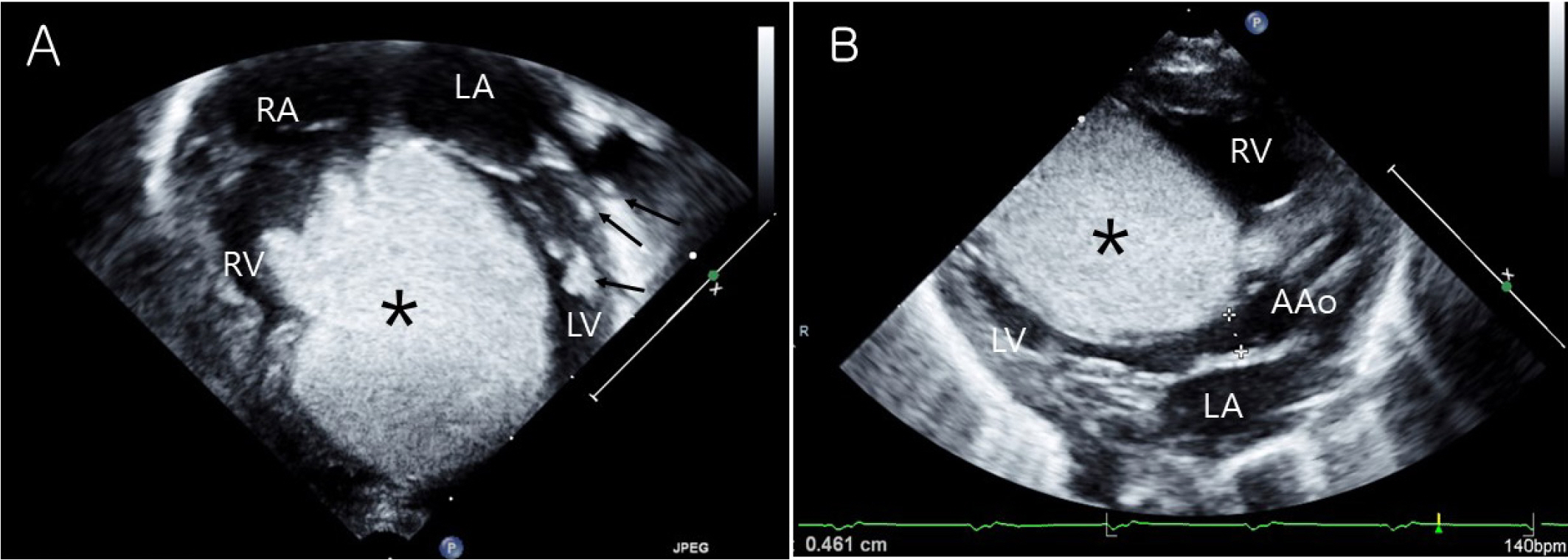
Fig. 2.
Brain ultrasound image in neonate with tuberous sclerosis. Multiple cortical and subcortical tuber (arrows) in fronto-parieto-occipital area are showed in (A) coronal view and (B) parasagittal view of right and left brain.
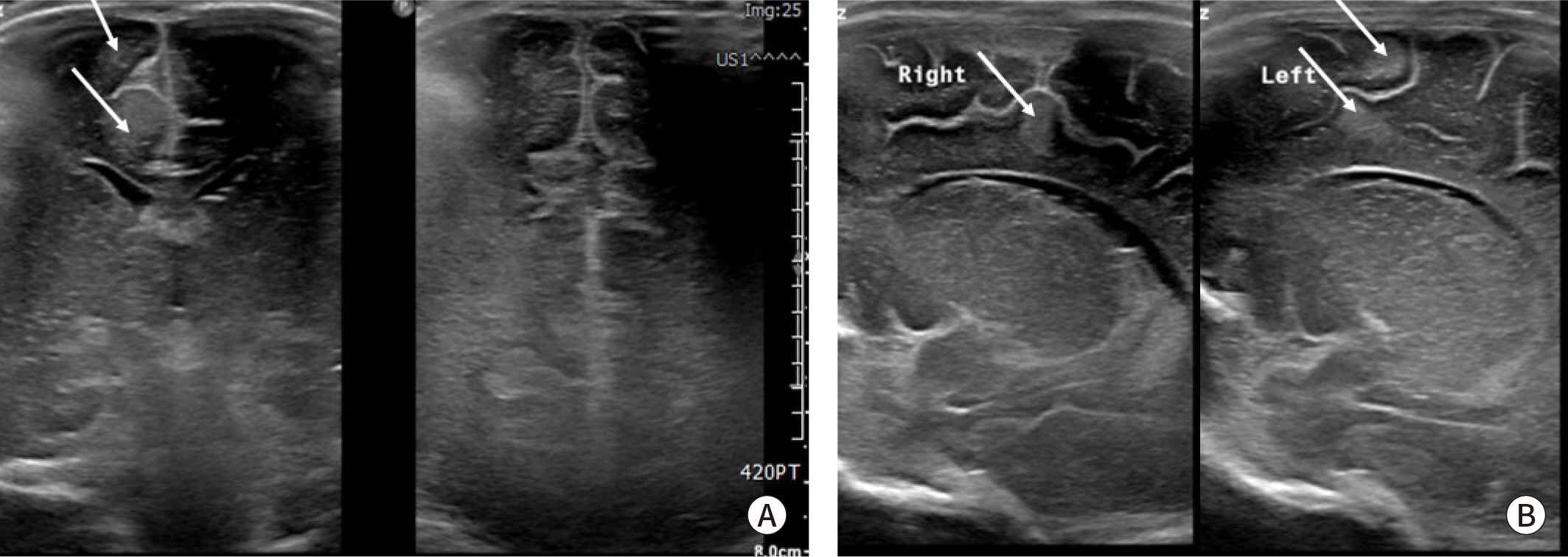
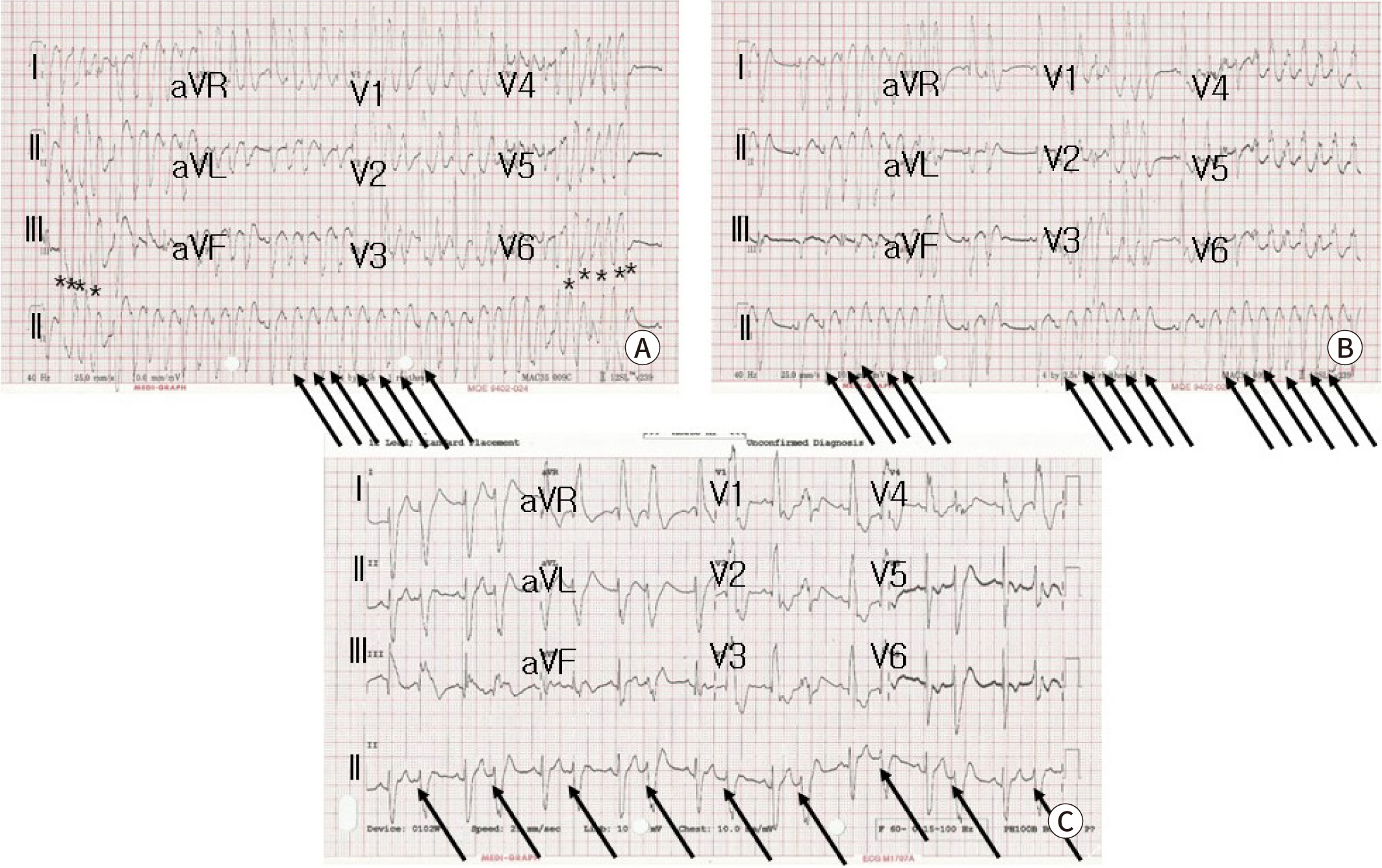
Fig. 3.
12 leads surface ECG showing wide QRS tachycardia. (A) Initial ECG shows atrial tachycardia (arrows) with nonsustained ventriculat tachycardia (asterisks). (B) After amiodarone infusion, nonsustained atrial tachycardia (arrows) and (C) frequent atial premature complex with bigeminy (arrows) are shown.
Discussion
Rhabdomyomas are the most common cardiac tumours diagnosed in fetuses and neonates.
Cardiac rhabdomyomas in neonates tend to regress spontaneously and are not usually operated upon. However, they may lead to arrhythmias, heart failure, and ventricular inflow/outflow tract obstruction in the neonatal period [
2]. If they become obstructive or hemodynamically compromised, causing life threatening complications, surgical resection is indicated. Because removing all cardiac masses from within the myocardium does more damage in neonate, surgeons resect only the obstructing intracavitary portions. In one series 10% cases of cardiac rhabdomyoma required surgical subtotal tumor excision [
3].
Cardiac rhabdomyomas are typically presented in the tuberous sclerosis in the newborn. Tuberous sclerosis is multisystemic disease caused by gene mutation of TSC1 or TSC2 that are part of m-TOR pathway. Multiple systemic harmatomatous growth is resulted from m-TOR activation. Recent studies have reported successful treatment of life-threatening cardiac rhabdomyoma in a newborn infants with m-TOR inhibitors. Therapy with m-TOR inhibitor has shown decreased the cardiac mass size relieving the ventricular outflow tract obstruction and improving the cardiac function when not eligible for resection or as alternative [
1,
4–
6].
In the case, we initially started m-TOR inhibitor because cardiac mass was inoperable lesion without definite ventricular outflow tract obstruction. During therapy, hemodynamic condition was stable and tachyarrhythmias were relatively well controlled by intravenous amiodarone infusion. However, patient was compromised as blood pressure suddenly dropped in day 10 of hospitalization.
Cardiac rhabdomyomas are frequently multiple, involving the both ventricular septal and free wall and also involve either atrium. A wide spectrum of clinical symptom especially, various cardiac arrhythmias can be associated with rhabdomyoma in pediatric patients [
1,
2]. Previous study reported that atrial and/or ventricular ectopic beats were seen in 24% of neonates and infants. And other critical arrhythmias, such as pre-excitation syndrome, refractory ectopic AT and incessant VT were demonstrated in cardiac rhabdomyomas [
1,
7,
8]. In the case, nonsustained VT was intermittently observed. So, it was not clearly documented on 12-lead ECG. Although ECG findings were limited, nonsustained VT showed positive QRS vectors in lead I, II, III, and V6. The suspected origin of VT is probably seen as the high septum area which was coincided with cardiac mass location. In fetal cardiac rhabdomayoma, pre-excitation syndrome was present in 80% of the fetuses and more common in fetal life than infants and children [
9]. The infantile pre-excitation is known to regress over the first year after birth [
10]. Although all types of arrhythmias have been described, ventricular tachyarrhythmias related with cardiac rhabdomyoma are rare than atrial tachyarrhythmias. In the study, multiple cardiac mass were mostly present in the ventricle but the majority of arrhythmias ware manifestated as atrial origin. Even though atrial tumor are not visible, undetected small abnormal atrial tissue with greater automaticity of atrial cells may be contributed to atrial tachyarrhythmias.
The majority of cardiac arrhythmias spontaneously healed or was gradually reduced of arrhythmia burden. In case of critical tachyarrhythmias, antiarrhythmic agents have been shown to control successfully. Although there is no clear first-line therapy for neonatal tachyarrhythmias, intravenous amiodarone is known to effective for neonatal supraventricular and ventricular dysarrhythmias for intracavitary occupying structural heart disease. Use of propranolol or digoxin for neonatal supraventricular tachyarrhythmias can be considered as maintenance therapy. Lidocaine is first-line therapy for wide QRS tachycardia of unknown mechanism [
11]. However, there are several reports of m-TOR inhibitor therapy about successful management of intractable rhabdomyoma-related tachyarrhythmias unresponsive to antiarrhythmic drugs [
7,
10]. Although atrial and ventricular arrhythmias were responsive to amiodarone and m-TOR inhibitor, tumor regression was unsatisfactory for 10 days in the case. The cause of death is presumed that ventricular outflow tract obstruction contributed to the hemodynamic instability but adverse effect of the drugs may be considered as other factor.







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download