This article has been
cited by other articles in ScienceCentral.
Author's summary
Despite improved clinical outcome of percutaneous coronary intervention (PCI) as development of coronary stent technology, stent-related complications have been a limitation in PCI. Since the most problematic in this context is stent thrombosis, one of the newer methodologies of the stent for overcoming this is to reduce the likelihood of thrombosis and increase biocompatibility. For this purpose, a stent of newer concepts was invented as application of biodegradable polymers and adoption of abluminal coating technique. We evaluated the efficacy and safety of this stent by comparing it with a stent that has been already studied by previous clinical trials.
Abstract
Background and objectives
To compare the safety and efficacy of a new everolimus-eluting stent with an abluminal-coated biodegradable polymer (Osstem Cardiotec Centum) with those of the Xience Alpine stent (Xience).
Methods
This randomized, prospective, multicenter, parallel-designed, single-blind trial was conducted among patients with myocardial ischemia undergoing percutaneous coronary intervention (PCI) from 21st September 2018 until 3rd July 2020. The primary efficacy endpoint was in-segment late lumen loss (LLL) at 270 days after the procedure and the primary safety endpoints were major adverse cardiac events (MACE), composite of cardiac death, myocardial infarction, and target lesion revascularization.
Results
We enrolled 121 patients and analyzed 113 patients who finished 270 days of follow-up for the primary efficacy endpoint. The mean age of the participants was 66.8 years. As for the primary efficacy endpoint, LLL of the Osstem Cardiotec Centum group was 0.09±0.13 mm and that of the Xience group was 0.12±0.14 mm (upper limit of 1-sided 95% confidence interval, 0.02; p for non-inferiority, 0.0084). This result demonstrates the non-inferiority of the Osstem Cardiotec Centum. As for the primary safety endpoint, MACE occurred in one patient (1.59% of the Xience group). Meanwhile, no MACE occurred in the Osstem Cardiotec Centum group.
Conclusions
The Osstem Cardiotec Centum is non-inferior to the Xience Alpine® stent and is confirmed to be safe. It could be safely and effectively applied to patients with coronary artery disease undergoing PCI.
Keywords: Coronary disease, Percutaneous coronary intervention, Drug-eluting stents, Coronary restenosis
INTRODUCTION
The coronary artery stent has reduced the incidence of acute complications after percutaneous coronary intervention (PCI) through technological developments. These complications include arterial recoil, dissection, and late neointimal hyperplasia.
1) Researchers have improved stent strut design and thickness, polymer coating and drug-elution to reduce in-stent restenosis. As a result, state-of-the-art coronary stents show excellent efficacy and safety.
2) The Xience Alpine stent has cobalt-chrome alloy for metallic structure and a strut thickness of 81 μm. It has a circumferential durable polymer that elutes everolimus for 4 months. The Xience™ drug-eluting stent (DES) has been proven to provide good clinical outcomes during and long after PCI.
3)4)5) We have chosen Xience Alpine stent as control stent since it is the same everolimus eluting stent as Osstem Cardiotec Centum stent, but has different stent structure profiles including adopted durable polymers and stent platform structure. Details of stent profiles were described at
Supplementary Table 2.
The Osstem Cardiotec Centum stent developed by Osstem Cardiotec Co. (Seoul, Korea) adopts polylactide, a biodegradable polymer, to reduce the likelihood of thrombosis and increase biocompatibility by preventing inflammatory reactions and peeling of the coating layer. It is made with a cobalt-chrome alloy for open cell structure and has a strut thickness, 85 μm.
The stent structure of the Osstem Cardiotec Centum has 2 major features regarding the cell type, number of cells, and connectors between stent cells: the open 6-cell structure and peak-to valley connector. While most other products have a 9-cell structure, this stent consists of 6 cells with an open-cell structure, resulting in a lower metal-to-artery ratio which can promote re-endothelialization.
The open-cell structure has lower radial strength than a closed-cell structure but has high flexibility and conformability. To overcome the low radial strength, the connectors of this stent were designed as peak-to-valley connectors, which are stronger than peak-to-peak connectors and have low stent foreshortening in the longitudinal direction. In addition, they were designed in a U-shape to increase the longitudinal strength. This can prevent stent migration that can occur when inserting equipment such as an intravascular imaging catheter.
The immunosuppressant, everolimus, was used as the drug of choice, based on clinical studies showing that the everolimus-eluting stent had a significantly lower incidence of late thrombosis compared to the paclitaxel-eluting stent and the sirolimus-eluting stent.
6) Osstem Cardiotec Centum uses an abluminal biodegradable coating, thereby improving the rate of re-endothelialization, and reducing the possibility of very late inflammation caused by polymers. The combination of this improved stent structure and biocompatible elements, such as biodegradable polymer and the abluminal biodegradable coating method, has not been studied in reference to clinical outcomes. The safety and efficacy of this stent was confirmed in a pre-clinical animal studies that was submitted to the Ministry of Food and Drug Safety, Republic of Korea. The aim of this clinical trial was to prove the non-inferior clinical efficacy and safety of Osstem Cardiotec Centum compared to the previously approved and widely used Xience Alpine (Abbott Laboratories, Chicago, IL, USA).
METHODS
Ethical statement
The study protocol was reviewed and approved by the Institutional Review Board (IRB) of Seoul National University Bundang Hospital (IRB No. E-1803/456-002) and the other recruiting institutions. This study was registered in the clinical trial list of Ministry at the Food and Drug Safety of Korea for medical device approval (registered No. 873). Subjects in this study voluntarily signed the consent form.
Study design
This clinical trial was a prospective, multicenter, parallel, single-blind, randomized clinical trial. We compared Osstem Cardiotec Centum versus Abbott's “Xience Alpine®” based on data collected for up to 270 days after PCI in 6 medical institutions of South Korea. The subjects were randomly assigned to one of the stents at a ratio of 1:1. Subjects visited for follow-up at 30, 180, and 270 days after the procedure, respectively. They were evaluated for efficacy and safety according to the schedule at each visit.
Data collection and study protocol
Subjects were enrolled if they met the inclusion/exclusion criteria during screening. At the screening visit, the baseline data were investigated and evaluated. Inclusion criteria included adults aged over 19 years who were suitable for PCI due to myocardial ischemia such as stable angina, unstable angina, and asymptomatic ischemia. The criteria also started that patients should have stenosis in the de novo lesion located in the native coronary artery (>50% diameter stenosis [DS] on coronary angiography and thrombolysis in myocardial infarction flow grade 1 or more, a diameter of 2.25 mm or more and 4.00 mm or less, and a lesion length of 34 mm or less during coronary angiography).
Patients were excluded if they had presented with acute myocardial infarction within a year from the enrolment, with experiencing cardiogenic shock, reduced left ventricular ejection fraction of less than 30%, restenosis lesions, lesion located on the saphenous vein graft, a history of bleeding tendency or blood clotting disorder, the expected life expectancy of 1 year or less due to accompanying disease, allergy or a contraindication to everolimus, aspirin, clopidogrel cobalt chromium, PLA/PLGA or contrast media, etc., total occlusion, a lesion of a blood vessel that had undergone other PCI procedures (balloon angiography, stent, cutting balloon atherectomy, etc.) or brachytherapy within 9 months (registration is possible if the procedure was performed on a vessel other than the target vessel.). It also excluded patients having a lesion with bifurcation or ostial lesion of >2 mm in diameter and occluded by 50% or more, a case in which a procedure/operation was scheduled to stop double antiplatelet therapy (DAPT) within 12 months after the procedure, or with a platelet count of <100,000 cells/mm3 or> 700,000 cells/mm3, a leukocyte count of <3,000 cells/mm3, a serum creatinine level ≥2.5 mg/dL, or on dialysis. Patients who had the lesion present in the left main coronary artery, were pregnant, or lactating mothers, planned to become pregnant within 12 months of the procedure, or disagreed with the use of appropriate contraception during the clinical trial were also excluded. Lastly, patients were excluded in the case of clinically significant abnormal values of hematological results and urinalysis, which are laboratory test items performed at the time of screening. or if they had reduced immunity.
The registered subjects were hospitalized on the day before or on the day of the scheduled procedure according to the institution's standard procedure and received pre-procedure treatment, including an aspirin and a P2Y12 inhibitor (clopidogrel, ticagrelor, or prasugrel) as DAPT. The subjects were discharged from the hospital according to the institution's standard procedure after PCI. The subjects were then hospitalized for 270 days after PCI for coronary angiography and follow-up data.
Percutaneous coronary intervention procedure
According to the judgment of the investigator and the method of use suggested by the manufacturer, a stent of the optimal size was selected in considering of the reference vessel diameter (RVD) and the length of the lesion. After pre-dilatation, the stent was delivered to the lesion using a balloon catheter system. After deploying the stent in the target lesion, the stent was expanded by applying a nominal pressure. For post-dilatation, a noncompliant balloon of the same size as the stent was used.
Quantitative coronary angiography
Quantitative angiographic analysis was performed by operators not involved in the intervention using CAAS® software (Pie Medical Imaging B.V, Maastricht, the Netherlands). A guiding catheter was used for magnification calibration as a standard index. In-segment/in-stent %DS, in-segment/in-stent minimal lumen diameter (MLD), and in-segment/in-stent reference lumen diameter were obtained from this system. These measurements were taken using the same end-diastolic angiographic frames acquired pre- and post-stent implantation. Acute gain was computed as the difference between the baseline MLD and the final post-stenting MLD of the procedure day. Late lumen loss (LLL) was computed as the difference between the final post-stenting MLD and the MLD found at follow-up angiography.
Study endpoints
The primary efficacy endpoint was in-segment LLL on follow-up coronary angiography at 270 days after PCI by an independent evaluator. In-segment was defined as a section including a segment corresponding to a location 5.0 mm away from both ends of the stent. LLL was calculated by subtracting the MLD at 270 days after PCI from the MLD immediately after stenting.
The secondary endpoints were target lesion failure (TLF) at 270 days after PCI and clinical success rate. TLF was defined as a composite of revascularization of the target lesion, target vessel myocardial infarction, and cardiac death. Clinical device success was defined as the successful application of the stent to the target lesion, the delivery system successfully removed, and residual stenosis found to be less than 30% on angiography. Clinical procedure success was defined as the case in which clinical device success was achieved and TLF did not occur for 24 hours after PCI.
Statistical analysis
This trial was conducted to prove the non-inferiority of the Osstem Cardiotec Centum to the Xience-Alpine stent with respect to the primary endpoint at 270 days. We assumed a SD of in-segment LLL (0.35 mm in each stent group derived from 5 recent clinical trials).
7)8)9)10) To verify the non-inferiority of Osstem Cardiotec Centum compared to Xience stents in this clinical trial, we set 0.195 mm as the non-inferiority margin of LLL at 9 months.
7) We calculated the appropriate sample size with a 1-sided significance level of 0.025 and 80% power. The sample size was 61 in each treatment arm in consideration of a 16% drop out rate. Sample size calculation in detail is represented in
Supplementary Data 1.
The primary endpoints and secondary endpoints were evaluated by intention-to-treat analysis. Additionally, in order to analyze safety issues that are generally reflected by actual biological and biochemical responses, the secondary endpoints for safety evaluation were analyzed using the as-treated set and these were described at
Supplementary Figure 1 and
Supplementary Table 3. Demographic data, basic characteristics, and safety data were obtained using the safety set.
Statistical significance was established at a 2-sided test significance level (α) of 5%. However, the non-inferiority test was performed using the 97.5% 1-sided confidence interval (CI; the upper limit of the 95% 2-sided CI). For normally distributed continuous data, the 2 sample t-test is used, whereas the Wilcoxon rank sum test was used for non-normally distributed continuous data. A chi-squared test was used for categorical, and when cells with an expected frequency of less than 5 were 20% or more of all cells, the significance was examined using Fisher's exact test. Descriptive statistics for LLL were presented in the Osstem Cardiotec Centum and Xience Alpine groups to analyze differences in LLL as the primary endpoint. Since a 95% 2-sided CI for the mean difference between the 2 groups (LLL mean in the test group − LLL mean in the Xience Alpine group) did not have normality on normality test, we had obtained the 95% CI of the median difference between the LLL values of the 2 groups based on the Hodges-Lehmann estimation as a nonparametric test. From this, if the upper limit of the 95% bilateral CI was smaller than the preset non-inferiority criterion (0.195),
7) the test device (the Osstem Cardiotec Centum) was judged to be non-inferior to the control device (Xience Alpine).
RESULTS
Study population
Among patients who had visited outpatient clinics in 6 institutions from September 21, 2018 to July 3, 2020, those who required PCI due to de novo lesions were screened. Out of a total of 125 subjects, we randomly assigned 59 subjects to the Osstem Cardiotec Centum group and 62 subjects to the Xience Alpine group after excluding 4 screening dropouts (
Figure 1). All randomized subjects were treated with the allocated stents except one subject who was randomly assigned to the Osstem Cardiotec Centum group; however, an error occurred, and hence, the Xience Alpine was applied. Therefore, 58 subjects were subjected to Osstem Cardiotec Centum while 63 subjects were subjected to the Xience Alpine stent.
Figure 1
Study flow.
*Immigration to abroad, inability to visit; †Small vessel size.
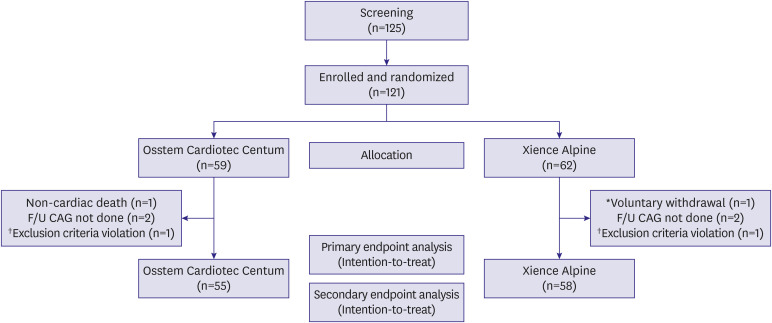
The number of subjects who failed to complete the follow-up until 270 days after the procedure and dropped out was 4 in the Osstem Cardiotec group and 4 in the Xience group, hence totaling to 8 subjects. Therefore, data from 55 subjects in the Osstem Cardiotec group and 58 subjects in the Xience group were analyzed with the intention-to-treat primary endpoint. All subjects completed the clinical follow-up for 1 year. Data from a total of 121 subjects (58 in the Osstem Cardiotec group and 63 in the Xience group as treated) were analyzed for safety endpoints.
Baseline characteristics
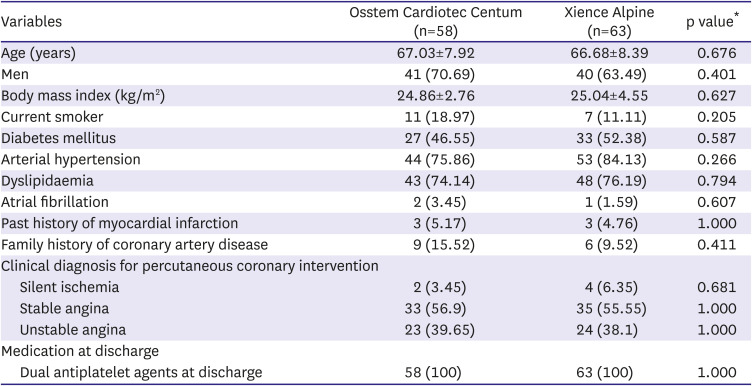
The demographic and clinical characteristics of the 121 subjects were well balanced between the 2 groups (
Table 1). The mean age was 67.03 in the Osstem Cardiotec group and 66.68±8.39 in the Xience group. The risk factors for coronary artery disease such as current smoking, diabetes mellitus, hypertension, and dyslipidemia were not different. The most clinical diagnoses for PCI were stable angina (59% in the Osstem Cardiotec group and 67% in the Xience group) and unstable angina (45% and 40%, respectively). DAPT was prescribed to all subjects in both groups.
Table 1
Baseline characteristics of the study population
|
Variables |
Osstem Cardiotec Centum (n=58) |
Xience Alpine (n=63) |
p value*
|
|
Age (years) |
67.03±7.92 |
66.68±8.39 |
0.676 |
|
Men |
41 (70.69) |
40 (63.49) |
0.401 |
|
Body mass index (kg/m2) |
24.86±2.76 |
25.04±4.55 |
0.627 |
|
Current smoker |
11 (18.97) |
7 (11.11) |
0.205 |
|
Diabetes mellitus |
27 (46.55) |
33 (52.38) |
0.587 |
|
Arterial hypertension |
44 (75.86) |
53 (84.13) |
0.266 |
|
Dyslipidaemia |
43 (74.14) |
48 (76.19) |
0.794 |
|
Atrial fibrillation |
2 (3.45) |
1 (1.59) |
0.607 |
|
Past history of myocardial infarction |
3 (5.17) |
3 (4.76) |
1.000 |
|
Family history of coronary artery disease |
9 (15.52) |
6 (9.52) |
0.411 |
|
Clinical diagnosis for percutaneous coronary intervention |
|
|
|
|
Silent ischemia |
2 (3.45) |
4 (6.35) |
0.681 |
|
Stable angina |
33 (56.9) |
35 (55.55) |
1.000 |
|
Unstable angina |
23 (39.65) |
24 (38.1) |
1.000 |
|
Medication at discharge |
|
|
|
|
Dual antiplatelet agents at discharge |
58 (100) |
63 (100) |
1.000 |
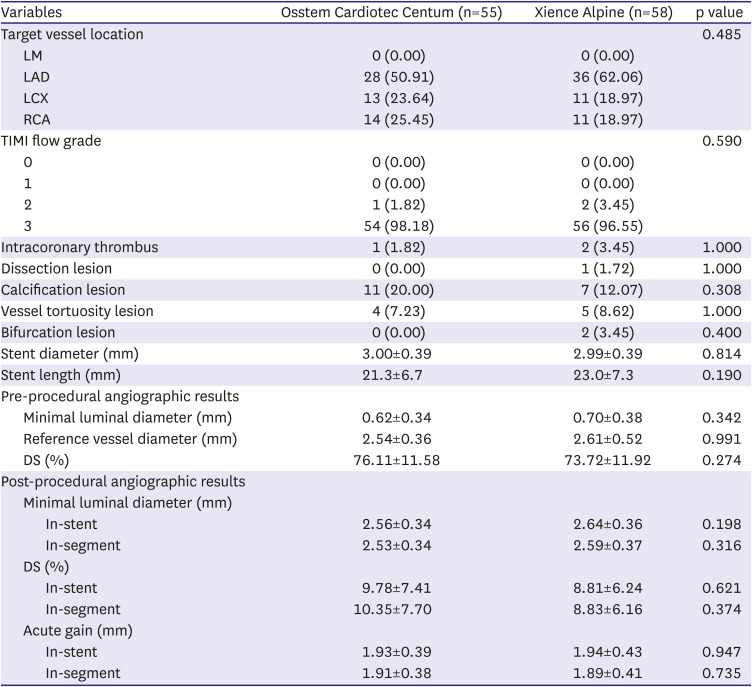
Lesion and procedure characteristics
Lesion and procedure characteristics of 113 subjects who underwent follow-up angiography are shown in
Table 2. The target lesion location and lesion complexity were not significantly different between the groups. The stent diameter and length were comparable between the groups without statistical significance.
Table 2
Lesion and procedure characteristics
|
Variables |
Osstem Cardiotec Centum (n=55) |
Xience Alpine (n=58) |
p value |
|
Target vessel location |
|
|
0.485 |
|
LM |
0 (0.00) |
0 (0.00) |
|
LAD |
28 (50.91) |
36 (62.06) |
|
LCX |
13 (23.64) |
11 (18.97) |
|
RCA |
14 (25.45) |
11 (18.97) |
|
TIMI flow grade |
|
|
0.590 |
|
0 |
0 (0.00) |
0 (0.00) |
|
1 |
0 (0.00) |
0 (0.00) |
|
2 |
1 (1.82) |
2 (3.45) |
|
3 |
54 (98.18) |
56 (96.55) |
|
Intracoronary thrombus |
1 (1.82) |
2 (3.45) |
1.000 |
|
Dissection lesion |
0 (0.00) |
1 (1.72) |
1.000 |
|
Calcification lesion |
11 (20.00) |
7 (12.07) |
0.308 |
|
Vessel tortuosity lesion |
4 (7.23) |
5 (8.62) |
1.000 |
|
Bifurcation lesion |
0 (0.00) |
2 (3.45) |
0.400 |
|
Stent diameter (mm) |
3.00±0.39 |
2.99±0.39 |
0.814 |
|
Stent length (mm) |
21.3±6.7 |
23.0±7.3 |
0.190 |
|
Pre-procedural angiographic results |
|
|
|
|
Minimal luminal diameter (mm) |
0.62±0.34 |
0.70±0.38 |
0.342 |
|
Reference vessel diameter (mm) |
2.54±0.36 |
2.61±0.52 |
0.991 |
|
DS (%) |
76.11±11.58 |
73.72±11.92 |
0.274 |
|
Post-procedural angiographic results |
|
|
|
|
Minimal luminal diameter (mm) |
|
|
|
|
|
In-stent |
2.56±0.34 |
2.64±0.36 |
0.198 |
|
|
In-segment |
2.53±0.34 |
2.59±0.37 |
0.316 |
|
DS (%) |
|
|
|
|
|
In-stent |
9.78±7.41 |
8.81±6.24 |
0.621 |
|
|
In-segment |
10.35±7.70 |
8.83±6.16 |
0.374 |
|
Acute gain (mm) |
|
|
|
|
|
In-stent |
1.93±0.39 |
1.94±0.43 |
0.947 |
|
|
In-segment |
1.91±0.38 |
1.89±0.41 |
0.735 |
Quantitative coronary angiography
Pre-procedural MLD and RVD were not significantly different between the groups and thus DS was comparable (
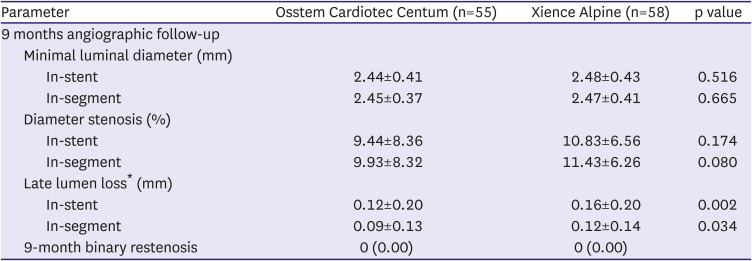
Table 2). Post-procedural MLD, DS and acute gain were not different between the groups. In the 9 months angiographic data, MLD and DS were similar between the groups (
Table 3). However, LLL of the Osstem Cardiotec group was 0.09±0.13 mm and that of the Xience group was 0.12±0.14 mm (p=0.034).
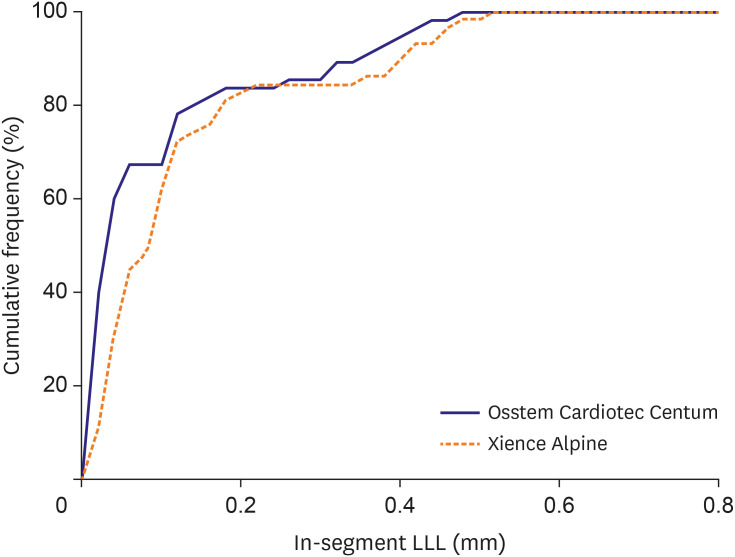
Figure 2 presents the cumulative frequency of in-segment of LLL. The average difference between the 2 groups was −0.03 mm (95% CI: −0.05, −0.01), and the upper limit of the CI was −0.01, which was found to be smaller than the non-inferiority margin of 0.195, which demonstrated the non-inferiority of the Osstem Cardiotec Centum stent.
Table 3
Quantitative coronary angiographic outcomes (analysis set)
|
Parameter |
Osstem Cardiotec Centum (n=55) |
Xience Alpine (n=58) |
p value |
|
9 months angiographic follow-up |
|
|
|
|
Minimal luminal diameter (mm) |
|
|
|
|
|
In-stent |
2.44±0.41 |
2.48±0.43 |
0.516 |
|
|
In-segment |
2.45±0.37 |
2.47±0.41 |
0.665 |
|
Diameter stenosis (%) |
|
|
|
|
|
In-stent |
9.44±8.36 |
10.83±6.56 |
0.174 |
|
|
In-segment |
9.93±8.32 |
11.43±6.26 |
0.080 |
|
Late lumen loss* (mm) |
|
|
|
|
|
In-stent |
0.12±0.20 |
0.16±0.20 |
0.002 |
|
|
In-segment |
0.09±0.13 |
0.12±0.14 |
0.034 |
|
9-month binary restenosis |
0 (0.00) |
0 (0.00) |
|
Figure 2
Cumulative graph of LLL compared 2 stents.
LLL = late lumen loss.
Clinical outcomes
The success rate of the device or procedure was 100% in both groups (
Table 4). As for the TLF, one ischemic target lesion revascularization occurred in the Xience group. In the Osstem Cardiotec group, one patient died due to exacerbation of underlying COPD and pneumoconiosis.
Table 4
Clinical outcomes
|
Variables |
Osstem Cardiotec Centum (n=55) |
Xience Alpine (n=58) |
|
Clinical device success |
55 (100) |
58 (100) |
|
Clinical procedure success |
55 (100) |
58 (100) |
|
Target lesion failure |
0 (0.00) |
1 (1.72) |
|
Death |
|
|
|
All cause death |
1 (1.82) |
0 (0.00) |
|
Cardiac death |
0 (0.00) |
0 (0.00) |
|
Non-cardiac death |
1 (1.82) |
0 (0.00) |
|
Target vessel-related myocardial infarction |
0 (0.0) |
0 (0.00) |
|
Any myocardial infarction |
0 (0.00) |
0 (0.00) |
|
Ischemia-driven target lesion revascularization |
0 (0.00) |
1 (1.72) |
|
Ischemia-driven target vessel revascularization |
0 (0.00) |
1 (1.72) |
|
Any repeat revascularization |
0 (0.00) |
1 (1.72) |
|
Stent thrombosis |
0 (0.0) |
0 (0.00) |
|
Patient-oriented composite endpoint |
1 (1.82) |
1 (1.72) |
|
Bleeding |
0 (0.00) |
0 (0.00) |
DISCUSSION
In this clinical trial to prove non-inferior clinical efficacy and safety, the Osstem Cardiotec Centum stent showed non-inferior LLL at 9 months after PCI compared to the Xience Alpine stent. It also demonstrated a good clinical device/procedural success rate and satisfactory 9-month outcomes in terms of TLF without safety issues.
With the introduction of DES, it has been possible to significantly reduce the occurrence of in-stent restenosis compared to the bare metal stents. However, DES implantation delayed vascular healing
11) and endothelialization of the stent strut,
12) which might be associated with late complications such as late stent thrombosis. Furthermore, durable polymers are thought to be associated with triggering the inflammatory process, which leads to stent thrombosis.
13)
To improve these problems and obtain better clinical outcomes, a new concept of the stent was introduced as the application of biodegradable polymers and the adoption of an abluminal coating technique. Based on clinical studies, biodegradable polymers have a significantly lower incidence of vascular inflammation than non-degradable polymers.
14)15) In the Osstem Cardiotec Centum stent, polylactide-D, a biodegradable polymer that is completely decomposed into water and carbon dioxide, was adopted. The abluminal coating method was applied to the stent struts, thereby improving the speed of endothelial cell formation, eliminating the polymer remaining in the stent after drug release, and reducing the likelihood of developing inflammation. These theoretical assumptions seem to actually improve the clinical efficacy of the Osstem Cardiotec Centum in terms of LLL as it showed a non-inferior result to the Xience stent in this study.
In addition, TLF occurred in one case (ischemia-driven target lesion revascularization) in the Xience group, but not in the Osstem Cardiotec group, although the number of subjects in this study was not large enough to evaluate the difference between groups in TLF, the safety of Osstem Cardiotec stent confirmed in this small clinical trial. The device and procedure success rate of the Osstem Cardiotec stent was confirmed to be 100.0%, which suggests a good device performance in relation to delivery, deployment, radial force and minimal residual stenosis.
In conclusion, the Osstem Cardiotec Centum was confirmed to be non-inferior to the Xience Alpine® stent in terms of LLL as well as the device success and procedure success rate. Therefore, the Osstem Cardiotec Centum stent could be safely and effectively applied in patients undergoing PCI. Long-term clinical efficacy and safety need to be evaluated in future larger-scale clinical trials or registries.








 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download