Abstract
This study aimed to investigate the association between Bacille Calmette-Guérin (BCG) vaccination and nontuberculous mycobacterial pulmonary disease (NTM-PD). Patients in the prospective NTM-PD cohort were matched to healthy controls to measure the association between BCG and NTM-PD development. The clinical course of NTM-PD patients was also evaluated to investigate the association between BCG and NTM-PD progression. BCG scars were not associated with NTM-PD development (adjusted odds ratio [OR], 2.04; 95% confidence interval [CI], 0.96–4.34) or progression (adjusted OR, 1.61; 95% CI, 0.92–2.81). In conclusion, BCG vaccination was not associated with the development or progression of NTM-PD.
Graphical Abstract
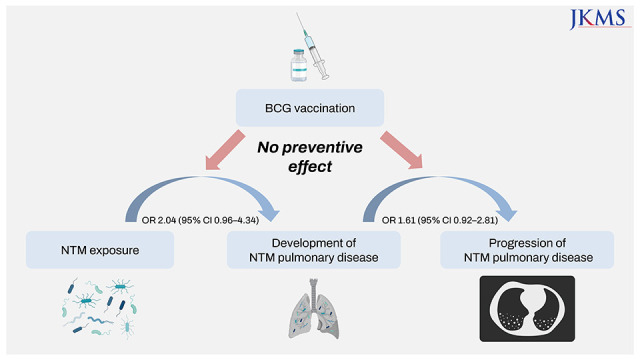
Bacille Calmette-Guérin (BCG), an attenuated strain of Mycobacterium bovis, has been used for centuries to prevent tuberculosis (TB).1 It has also been reported that BCG has beneficial effects against other respiratory infections through “trained immunity”.23 However, whether BCG prevents nontuberculous mycobacterial pulmonary disease (NTM-PD) remains uncertain. In mice, BCG has been shown to induce cross-protective T cell immunity against NTM such as M. avium and M. abscessus.4 In humans, BCG reduces the risk of NTM lymphadenitis and Buruli ulcer.15 The rapid increase in childhood NTM infection after BCG coverage drop also supports the protective effect of BCG against NTM.6 However, the effect of BCG on NTM-PD has not yet been evaluated. In this study, we investigated the association between BCG vaccination and the development and progression of NTM-PD.
Patients who were diagnosed with NTM-PD between July 1, 2011 and December 31, 2020 and participated in a prospective NTM-PD cohort of Seoul National University Hospital (NCT01616745) (NTM-PD cohort) were included in this study. To investigate the association between BCG vaccination and the development of NTM-PD (study 1), we conducted a case-control study. Age (± 3 years) and sex-matched (1:1) controls without abnormality in chest X-ray were randomly selected from participants of ‘Controls for Respiratory Disease Study (NCT03120481),’ which is another prospective cohort including healthy controls. A BCG scar, which is a surrogate marker of BCG vaccination, was identified by experienced nurses. Thereafter, to investigate the impact of BCG vaccination on the progression of NTM-PD (study 2), the clinical courses and outcomes of patients from the NTM-PD cohort were evaluated (Supplementary Fig. 1). The progression of NTM-PD was defined as the initiation of anti-mycobacterial treatment, which was determined at the discretion of duty physicians based on patients’ symptoms and radiographic aggravation. Microbiological cure was defined as a case in which respiratory specimen culture was negative at least three times consecutively after antibiotic administration and maintained until the end of treatment.7 Conditional and unconditional logistic regression with covariates were used to analyse the effect of BCG vaccination on NTM-PD development and progression, respectively. The initiation of antibiotic treatment was considered to be the progression of NTM-PD. All statistical analyses were performed using STATA version 17.0 (College Station, TX, USA).
During the study period, 499 patients with NTM-PD were enrolled in the study. Their median age was 63 years (interquartile range [IQR], 56–70), and 324 patients (64.9%) were women. BCG scars were identified in 409 patients (82.0%). The patients with BCG scars were younger than those without BCG scars, and there were more women. Detailed characteristics are provided in Supplementary Table 1.
Of the 499 patients with NTM-PD, 369 patients were matched one-to-one to an equal number of patients in the control group. In the unadjusted model, BCG scars were associated with the development of NTM-PD (crude odds ratio [OR], 1.88; 95% confidence interval [CI], 1.30–2.73). However, after adjusting for body mass index (BMI), previous TB, and smoking, BCG scars were not associated with the development of NTM-PD (adjusted OR, 2.04; 95% CI, 0.96–4.34) (Table 1).
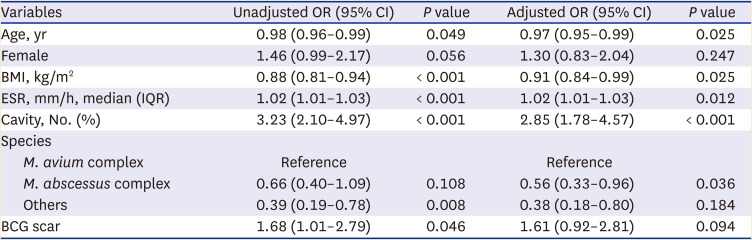
The patients who were enrolled in the NTM-PD cohort were followed up for a median duration of 63 months (IQR, 39–89). Among 409 patients with BCG scars, 155 patients (37.9%) initiated antibiotic treatment, while 24 out of 90 patients without BCG scars (26.7%) initiated antibiotic treatment (P = 0.028). The microbiological cure rate was 25.8% for patients with BCG scars and 25.0% for patients without BCG scars (P > 0.999) (Supplementary Table 2). High erythrocyte sediment rate (adjusted OR, 1.02; 95% CI, 1.01–1.03) and presence of cavities (adjusted OR, 2.85; 95% CI, 1.78–4.57) at the time of enrolment predicted initiation of antibiotic treatment, while age (adjusted OR, 0.97; 95% CI, 0.95–0.99) and BMI (adjusted OR, 0.91; 95% CI, 0.84–0.99) were inversely associated with initiating treatment. A BCG scar was not associated with the initiation of antibiotic treatment (adjusted OR, 1.61; 95% CI, 0.92–2.81) (Table 2).
In our study, BCG vaccination was not associated with the development and progression of NTM-PD. In study 1, the age- and sex-matched case-control study showed that BCG scars were not associated with the development of NTM-PD. In study 2, BCG vaccination did not affect the outcomes of NTM-PD. Possible explanations for the ineffectiveness of BCG against NTM-PD are as follows: First, the effect of BCG on NTM may vary according to clinical manifestations. BCG was more effective in preventing TB meningitis and military TB than preventing pulmonary TB.8 Likewise, BCG might be ineffective against NTM-PD, although some studies have shown the protective effects of BCG against NTM lymphadenitis or disseminated NTM infection.5 Second, the effect of BCG on NTM could increase over time. The protective effects of BCG against infectious organisms other than TB are thought to be induced by trained immunity, which confers nonspecific immune memory to innate immune responses by epigenetic reprogramming of immune cells.3 However, how long this trained immunity is maintained is unknown. In fact, most of the patients with NTM-PD in this study were older than 60 years. Therefore, the possible protective effects of BCG against NTM-PD may not be applied in this study.
This is the first study to analyse the role of BCG in NTM-PD. Previous studies have mainly focused on NTM infections other than pulmonary diseases.59 However, the most common presentation of NTM infection is pulmonary disease, and its prevalence has rapidly increased during the last decade.10 Therefore, it is timely and meaningful to investigate the effects of BCG on NTM-PD. Based on the prospective cohort, we showed that BCG was not protective against the progression and development of NTM-PD.
Notes
Author Contributions:
Conceptualization: Kwak N, Hwang HW, Yim JJ, Lee CH.
Data curation: Kwak N, Hwang HW.
Formal analysis: Kwak N, Hwang HW, Lee CH.
Investigation: Kwak N, Hwang HW, Kim HJ, Lee HW, Yim JJ, Lee CH.
Methodology: Kwak N, Hwang HW, Kim HJ, Lee HW, Yim JJ, Lee CH.
Software: Lee CH.
Validation: Kwak N, Lee CH.
Writing - original draft: Kwak N, Hwang HW, Kim HJ, Lee HW, Yim JJ, Lee CH.
Writing - review & editing: Kwak N, Hwang HW, Kim HJ, Lee HW, Yim JJ, Lee CH.
References
1. Mangtani P, Abubakar I, Ariti C, Beynon R, Pimpin L, Fine PE, et al. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin Infect Dis. 2014; 58(4):470–480. PMID: 24336911.

2. Moorlag SJ, Arts RJ, van Crevel R, Netea MG. Non-specific effects of BCG vaccine on viral infections. Clin Microbiol Infect. 2019; 25(12):1473–1478. PMID: 31055165.

3. Netea MG, Quintin J, van der Meer JW. Trained immunity: a memory for innate host defense. Cell Host Microbe. 2011; 9(5):355–361. PMID: 21575907.

4. Abate G, Hamzabegovic F, Eickhoff CS, Hoft DF. BCG Vaccination Induces M. avium and M. abscessus Cross-Protective Immunity. Front Immunol. 2019; 10:234. PMID: 30837992.
5. Zimmermann P, Finn A, Curtis N. Does BCG vaccination protect against nontuberculous mycobacterial infection? A systematic review and meta-analysis. J Infect Dis. 2018; 218(5):679–687. PMID: 29635431.

6. Kontturi A, Soini H, Ollgren J, Salo E. Increase in childhood nontuberculous mycobacterial infections after Bacille Calmette-Guérin coverage drop: a nationwide, population-based retrospective study, Finland, 1995–2016. Clin Infect Dis. 2018; 67(8):1256–1261. PMID: 29584893.

7. van Ingen J, Aksamit T, Andrejak C, Böttger EC, Cambau E, Daley CL, et al. Treatment outcome definitions in nontuberculous mycobacterial pulmonary disease: an NTM-NET consensus statement. Eur Respir J. 2018; 51(3):1800170. PMID: 29567726.

8. Colditz GA, Brewer TF, Berkey CS, Wilson ME, Burdick E, Fineberg HV, et al. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA. 1994; 271(9):698–702. PMID: 8309034.

9. Cunha SS, Alexander N, Barreto ML, Pereira ES, Dourado I, Maroja MF, et al. BCG revaccination does not protect against leprosy in the Brazilian Amazon: a cluster randomised trial. PLoS Negl Trop Dis. 2008; 2(2):e167. PMID: 18270542.

10. Lee H, Myung W, Koh WJ, Moon SM, Jhun BW. Epidemiology of nontuberculous mycobacterial infection, South Korea, 2007–2016. Emerg Infect Dis. 2019; 25(3):569–572. PMID: 30789139.

SUPPLEMENTARY MATERIALS
Supplementary Table 1
Baseline characteristics of patients with nontuberculous mycobacterial pulmonary disease according to BCG scars
Supplementary Table 2
Outcomes of patients with nontuberculous mycobacterial pulmonary disease according to BCG scars
Table 1
The effects of BCG vaccination on the development of nontuberculous mycobacterial pulmonary disease (369 pairs) in the conditional logistic regression model
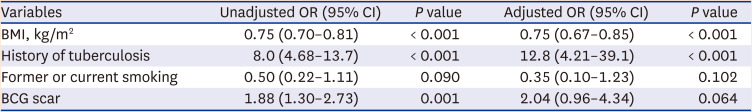
Table 2
The effects of BCG vaccination on the treatment initiation for nontuberculous mycobacterial pulmonary disease in the multivariable unconditional logistic regression model (N = 499)




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download