Abstract
The emergence and spread of antimicrobial resistance threatens the successful treatment of invasive pneumococcal disease (IPD). We report a case of disseminated pneumococcal infection with meningitis, spondylitis, and septic arthritis caused by an extremely drug-resistant strain of Streptococcus pneumoniae, which was resistant to at least one agent in all classes but was sensitive to vancomycin and linezolid. The patient was treated successfully using intravenous vancomycin (1 g every 12 h) and shoulder surgery. The serotype of this isolate was 15A, a non-vaccine type, and multi-locus sequence typing (MLST) revealed ST8279. MLST analysis and antimicrobial susceptibility test revealed that the strain, SMC1710-32, belonged to ST8279, with the same molecular characteristics with drug susceptibility as extensively drug-resistant (XDR) clone 11A-ST8279 in the previous studies. These XDR pneumococcal strains, serotype 11A and 15A possessed identical molecular characteristics including multiple mutated genes involved in very-high-level resistance to various drugs. The difference in serotype was due to large scale recombination for serotype switching. Further surveillance and investigation of the serotype distribution and genotypes of XDR strains are essential to prevent their spread.
Streptococcus pneumoniae causes several non-invasive and invasive bacterial diseases, including otitis media, pneumonia, and meningitis. The emergence and spread of antimicrobial resistance have threatened the successful treatment of invasive pneumococcal disease (IPD). Here, we report a case of acute disseminated infection caused by extensively drug-resistant (XDR) S. pneumoniae.
A 65-year-old woman who had been in a private orthopedic clinic for three weeks was admitted to the emergency department of Samsung Medical Center (SMC), Seoul, Republic of Korea on 29 September 2017 with a high-grade fever, chill, myalgia, and urinary discomfort of 3 day’s duration. She had received chemotherapy for the past 1 year due to multiple myeloma with renal and cardiac amyloidosis at SMC until 7 September 2017. She had not received either 13-valent pneumococcal conjugate vaccine (PCV13) or 23-valent pneumococcal polysaccharide vaccine (PPV23).
On 7 September, the patient visited a private clinic due to back pain after a fall. At the private clinic, she was diagnosed with L2 compression fracture and underwent vertebroplasty on 22 September 2017. On admission to our hospital, she was febrile (38.7℃), with a pulse rate of 103 beats/min, blood pressure of 113/79 mmHg, and a respiratory rate of 21 breaths/min. Initial physical examination showed left shoulder tenderness.
Laboratory tests showed 5,290 leukocytes/µL (segment neutrophil 85%), hemoglobin at 9.1 g/dL, and platelets at 166,000/µL. Other laboratory values included serum blood urea nitrogen (BUN) at 36.6 mg/dL, creatinine at 2.91 mg/dL, glucose at 267 mg/dL, CRP at 21.9 mg/dL, procalcitonin at 16.48 ng/mL, and lactic acid at 3.5 mM/L. Her chest radiograph showed no active lung lesions.
Cefazolin (2g IV q12h) was empirically administered based on a diagnosis of septic arthritis of the shoulder. The day after admission, blood cultures grew Gram-positive cocci in chains, and vancomycin (1g IV q12h) was added due to persistent fever. However, she subsequently developed mental deterioration with delirious features, and had no verbal output. Brain CT showed diffuse brain atrophy and spinal tapping was performed. Clear, serous fluid was drained and CSF analysis showed WBC count 960/µL with PMN 69%, which was consistent with bacterial meningitis. Unfortunately, culture, protein, and glucose were not tested due to an insufficient amount of fluid. Thus, we exchanged cefazolin with ceftriaxone (2g IV q12h), and vancomycin was continued.
The patient’s confused mentality improved gradually after starting ceftriaxone and vancomycin, and initial blood culture revealed S. pneumoniae, which was resistant to all antibiotics except tigecycline, vancomycin, and linezolid (Table 1). We discontinued ceftriaxone and the use of vancomycin was maintained. The follow-up blood cultures at 3 day after vancomycin use were negative. After mental recovery, the patient complained of back and left shoulder pain. MRI of the left shoulder and lumber spine was performed with gadolinium enhancement on 15 October. Spine MRI showed L5-S1 intervertebral disc signal intensity, with rim enhancing fluid collection at the anterior epidural and prevertebral area, suggesting infectious spondylodiscitis and anterior epidural and prevertebral abscess formation (Fig. 1A). The shoulder MRI showed rim enhancing fluid collection in the glenohumeral joint space, subcoracoid, and subacromial-deltoid bursa, which was suspicious of inflammatory condition, suggesting septic arthritis of shoulder (Fig. 1B). Because she had both meningitis and arthritis, we examined her presence of infective endocarditis. A heart murmur was examined and transthoracic echocardiogram was performed. There was no heart murmur on auscultation and there was no evidence of vegetation or other sign of infective endocarditis on echocardiogram.
The patient underwent incision and debridement of the left shoulder. We observed hyperemic capsule infection, bursitis, bursal hypertrophy, and granulation tissue infection during surgery. She underwent bursectomy, massive debridement, and partial release of the coracoacromial ligament. Gram staining and cultures of tissue and fluid became negative after vancomycin treatment over two-weeks. Detection of bacterial rDNA was not performed. Follow-up MRI of spine and shoulder was performed after five and six months, respectively. The abscess of spine MRI was disappeared and the enhancement of shoulder MRI was also improved. Clinically, she was diagnosed with disseminated infection and meningitis, arthritis, and spondylitis caused by S. pneumoniae.
The microbiological and molecular features of pneumococcal isolate, SMC1710-32 were described in our previous study [1]. Briefly, this isolate was serotyped as 15A and clonally belonged to ST8279, a double-locus variant of the pneumococcal Spain9V-3 ST156 international clone. Antimicrobial susceptibility of this isolate showed XDR profile, which is defined as non-susceptibility to all tested antimicrobial agents but for tigecycline, vancomycin, and linezolid. Multiple genetic mutations associated with XDR were retrieved from the data of whole genome sequencing of the isolate which we had recently performed (GenBank accession number CP025838) [1].
The emergence and spread of antimicrobial resistance threaten the successful treatment of IPD. More recently, the emergence of XDR S. pneumoniae has been reported in Korea [2-5]. Several case reports have described patients with disseminated pneumococcal infections, including an outbreak of multidrug-resistant (MDR) S. pneumoniae [6]. In the present study, we describe a case of bacteremic meningitis with dissemination to the lumbar spine and shoulder, caused by an XDR S. pneumoniae isolate, not just by MDR.
Since the introduction of the 7-valent pneumococcal conjugate vaccine (PCV7), the increase in the prevalence of serotypes not included in PCV7 has been observed worldwide, mainly in serotype 19A [7-9]. It has been replaced by PCV13, which targets six additional serotypes (1, 5, 7F, 3, 6A and 19A) [10]. Since the introduction of pneumococcal vaccines, these trends were continued with persistent prevalence of 19F and 19A with a noteworthy increase of certain non-PCV13 serotypes [11]. The pneumococcal isolate in this study was serotype 15A. It has been reported that serotype 15A is one of the most prevalent non-vaccine serotypes among S. pneumoniae isolates from the nasopharynx [12], as well as isolates from patients with invasive diseases [13].
Multilocus sequence typing analysis and antimicrobial susceptibility tests revealed that the isolate SMC1710-32 belonged to ST8279 and had the same molecular characteristics regarding drug susceptibility as XDR clone 11A-ST8279 as observed in previous studies [3,4,14]. Previously, we reported that several isolates of S. pneumoniae serotype 11A belonging to ST8279 exhibited XDR phenotypes. These XDR pneumococcal strains, serotypes 11A and 15A, possessed identical molecular characteristics: their genotype and antibiotic profiles had identical mutation patterns within resistance-determinant genes, except for the serotype. In our recent study, we reported this serotype difference was due to large scale recombination for serotype switching [1]. At initial, we did not know the differences in capsular locus regions between serotype 15A and 11A XDR strains. However, a comparative genomic approach using whole genome sequencing elucidated that the genome sequences of SMC1710-32 and 11A XDR strain (SMC1205-93) showed genetically identical genomes except for two regions; one was the cps locus and the other was the region flanked by pflB and radD.
Serotype changes through capsular switching have been reported as one of the causes of increased antibiotic resistance in pneumococcal isolates in several studies [15-17]. The mechanisms of XDR isolate with specific serotype causing multiple severe complications have not been fully elucidated. However, it is notable that the emergence of 15A XDR isolate via capsular switching from 11A XDR isolate, which have been increasingly isolated in South Korea, caused multiple severe complications in clinical setting. Emergence and clonal expansion of XDR pneumococcal strains complicates treatment and increases likelihood for severe outcomes because of treatment failure. Although only a single case was found, the emergence of XDR pneumococci via capsular switching should be carefully monitored and strategies to prevent its spread are strongly warranted. Additionally, the XDR pneumococcal isolate reported in this study comprises highly antimicrobial-resistant clones that are not covered by current vaccines and the increased incidence would continue under the current vaccine pressure. In fact, serotype 15A was found to be one of the most common non-vaccine serotypes among adult population in South Korea [18].
In conclusions, we reported a case of acute disseminated infection caused by XDR S. pneumoniae serotype 15A. As the dissemination of highly antimicrobial resistant clones, especially non-vaccine type, poses a great concern for public health. Further surveillance and investigation for serotype distribution and genotype of these XDR strains may be necessary to prevent its spread.
ACKNOWLEDGEMENTS
Bacterial isolates were obtained from the Asian Bacterial Bank (ABB) of the Asia Pacific Foundation for Infectious Diseases (APFID). This research was supported by Basic Science Research Program through NRF funded by the Ministry of Education (Grant no. 2018R1D1A1B07041096).
REFERENCES
1. Yang Baek J, Kim SH, Kang CI, Chung DR, Peck KR, Song JH, et al. 2018; Emergence of an extensively drug-resistant (XDR) Streptococcus pneumoniae serotype 15A by capsular switching. Int J Med Microbiol. 308:986–9. DOI: 10.1016/j.ijmm.2018.08.004. PMID: 30143394.

2. Kang CI, Baek JY, Jeon K, Kim SH, Chung DR, Peck KR, et al. 2012; Bacteremic pneumonia caused by extensively drug-resistant Streptococcus pneumoniae. J Clin Microbiol. 50:4175–7. DOI: 10.1128/JCM.01642-12. PMID: 23052301. PMCID: PMC3502956.

3. Baek JY, Kim SH, Kang CI, Chung DR, Peck KR, Ko KS, et al. 2016; Prevalence of antimicrobial resistant Streptococcus pneumoniae serotype 11A isolates in Korea, during 2004-2013, due to the increase of multidrug-resistant clone, CC166. Infect Genet Evol. 38:122–5. DOI: 10.1016/j.meegid.2015.12.018. PMID: 26733441.

4. Cho SY, Baek JY, Kang CI, Kim SH, Ha YE, Chung DR, et al. 2014; Extensively drug-resistant Streptococcus pneumoniae, South Korea, 2011-2012. Emerg Infect Dis. 20:869–71. DOI: 10.3201/eid2005.131371. PMID: 24750694. PMCID: PMC4012805.
5. Golden AR, Rosenthal M, Fultz B, Nichol KA, Adam HJ, Gilmour MW, et al. 2015; Characterization of MDR and XDR Streptococcus pneumoniae in Canada, 2007-2013. J Antimicrob Chemother. 70:2199–202. DOI: 10.1093/jac/dkv107. PMID: 25921512.
6. Nuorti JP, Butler JC, Crutcher JM, Guevara R, Welch D, Holder P, et al. 1998; An outbreak of multidrug-resistant pneumococcal pneumonia and bacteremia among unvaccinated nursing home residents. N Engl J Med. 338:1861–8. DOI: 10.1056/NEJM199806253382601. PMID: 9637804.

7. Naucler P, Galanis I, Morfeldt E, Darenberg J, Örtqvist Å, Henriques-Normark B. 2017; Comparison of the impact of pneumococcal conjugate vaccine 10 or pneumococcal conjugate vaccine 13 on invasive pneumococcal disease in equivalent populations. Clin Infect Dis. 65:1780–9. Erratum in: Clin Infect Dis 2019;68:534. DOI: 10.1093/cid/cix685. PMID: 29020171. PMCID: PMC5848315.

8. Kim SH, Song JH, Chung DR, Thamlikitkul V, Yang Y, Wang H, et al. 2012; Changing trends in antimicrobial resistance and serotypes of Streptococcus pneumoniae isolates in Asian countries: an Asian Network for Surveillance of Resistant Pathogens (ANSORP) study. Antimicrob Agents Chemother. 56:1418–26. DOI: 10.1128/AAC.05658-11. PMID: 22232285. PMCID: PMC3294909.

9. Cho EY, Choi EH, Kang JH, Kim KH, Kim DS, Kim YJ, et al. 2016; Early changes in the serotype distribution of invasive pneumococcal isolates from children after the introduction of extended-valent pneumococcal conjugate vaccines in Korea, 2011-2013. J Korean Med Sci. 31:1082–8. DOI: 10.3346/jkms.2016.31.7.1082. PMID: 27366006. PMCID: PMC4901000.

10. Alicino C, Paganino C, Orsi A, Astengo M, Trucchi C, Icardi G, et al. 2017; The impact of 10-valent and 13-valent pneumococcal conjugate vaccines on hospitalization for pneumonia in children: a systematic review and meta-analysis. Vaccine. 35:5776–85. DOI: 10.1016/j.vaccine.2017.09.005. PMID: 28911902.

11. Kim SH, Chung DR, Song JH, Baek JY, Thamlikitkul V, Wang H, et al. 2020; Changes in serotype distribution and antimicrobial resistance of Streptococcus pneumoniae isolates from adult patients in Asia: emergence of drug-resistant non-vaccine serotypes. Vaccine. 38:6065–73. DOI: 10.1016/j.vaccine.2019.09.065. PMID: 31590932.

12. Cohen R, Levy C, Bingen E, Koskas M, Nave I, Varon E. 2012; Impact of 13-valent pneumococcal conjugate vaccine on pneumococcal nasopharyngeal carriage in children with acute otitis media. Pediatr Infect Dis J. 31:297–301. DOI: 10.1097/INF.0b013e318247ef84. PMID: 22330166.

13. Richter SS, Heilmann KP, Dohrn CL, Riahi F, Beekmann SE, Doern GV. 2009; Changing epidemiology of antimicrobial-resistant Streptococcus pneumoniae in the United States, 2004-2005. Clin Infect Dis. 48:e23–33. DOI: 10.1086/595857. PMID: 19115971.
14. Choi MJ, Noh JY, Cheong HJ, Kim WJ, Kim MJ, Jang YS, et al. 2019; Spread of ceftriaxone non-susceptible pneumococci in South Korea: long-term care facilities as a potential reservoir. PLoS One. 14:e0210520. DOI: 10.1371/journal.pone.0210520. PMID: 30699137. PMCID: PMC6353129.

15. Nakano S, Fujisawa T, Ito Y, Chang B, Matsumura Y, Yamamoto M, et al. 2018; Spread of meropenem-resistant Streptococcus pneumoniae serotype 15A-ST63 clone in Japan, 2012-2014. Emerg Infect Dis. 24:275–83. Erratum in: Emerg Infect Dis 2018;24:1164. DOI: 10.3201/eid2402.171268. PMID: 29350141. PMCID: PMC5782878.
16. Chiba N, Murayama SY, Morozumi M, Iwata S, Ubukata K. 2017; Genome evolution to penicillin resistance in serotype 3 Streptococcus pneumoniae by capsular switching. Antimicrob Agents Chemother. 61:e00478–17. DOI: 10.1128/AAC.00478-17. PMID: 28630198. PMCID: PMC5571339.

17. Makarewicz O, Lucas M, Brandt C, Herrmann L, Albersmeier A, Rückert C, et al. 2017; Whole genome sequencing of 39 invasive Streptococcus pneumoniae sequence type 199 isolates revealed switches from serotype 19A to 15B. PLoS One. 12:e0169370. DOI: 10.1371/journal.pone.0169370. PMID: 28046133. PMCID: PMC5207522.

18. Lee S, Kim SH, Park M, Bae S. 2013; High prevalence of multiresistance in levofloxacin-nonsusceptible Streptococcus pneumoniae isolates in Korea. Diagn Microbiol Infect Dis. 76:227–31. DOI: 10.1016/j.diagmicrobio.2013.02.032. PMID: 23623384.

Fig. 1
(A) L-spine MRI showed high signal intensity at the L5-S1 intervertebral disc space, with rim enhancing fluid collection at the anterior epidural space and prevertebral area (arrows), and L5-S1 opposite endplate enhancement, suspicious of infectious spondylodiscitis and combined epidural abscess, resulting in canal compromise and right S1 nerve root compression. (B) Shoulder MRI with enhancement showed rim enhancing fluid collection (arrow) in the glenohumeral joint space, subcoracoid, and subacromial-deltoid bursa, suspicious of an inflammatory condition such as infection.
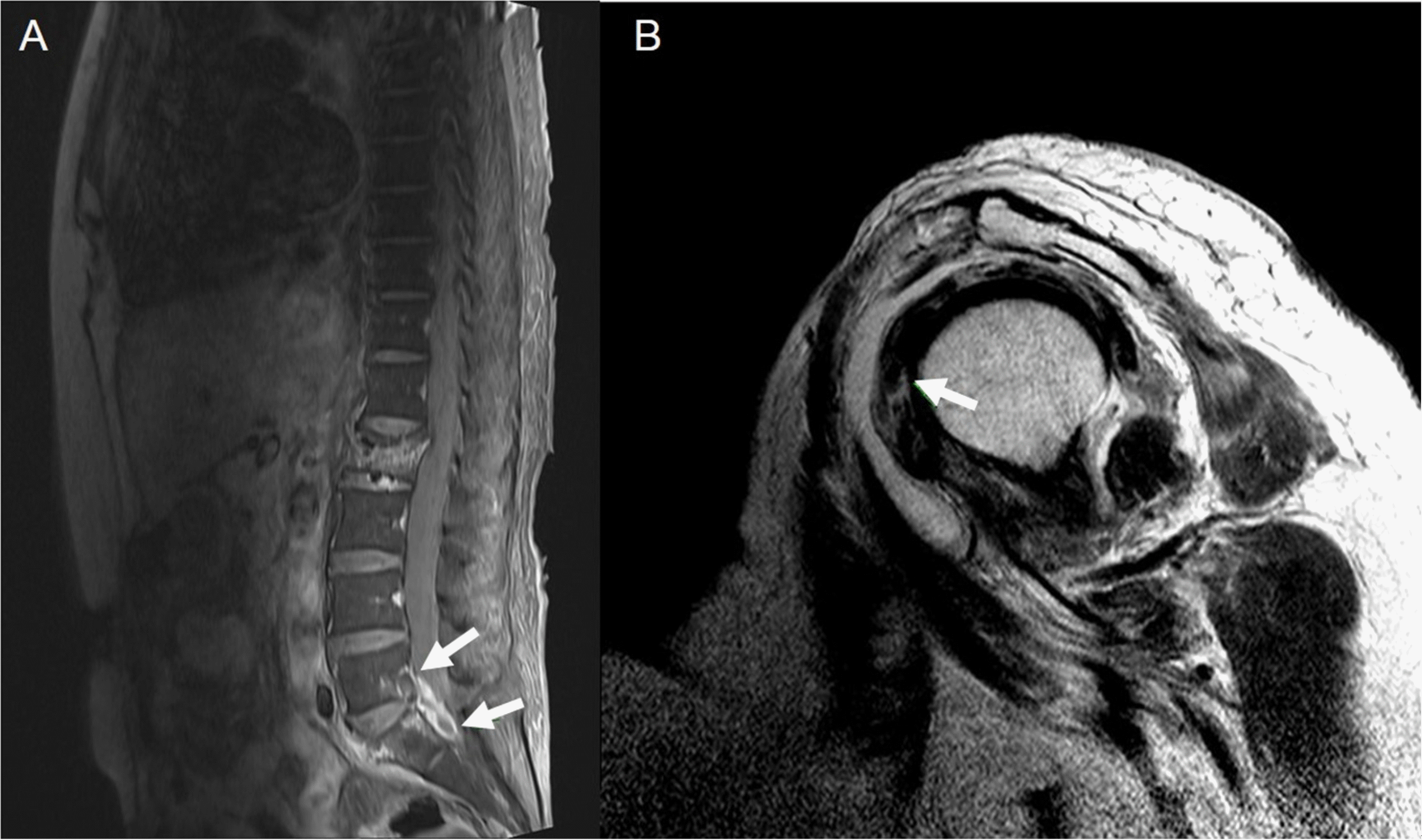
Table 1
Antimicrobial susceptibility by BITEK of S. pneumoniae isolate SMC1710-32
| Antimicrobial agent | MIC (µg/mL) | Susceptibility* |
|---|---|---|
| Penicillin | 8 | R |
| Amoxicillin | 32 | R |
| Amoxicillin/clavulanate | 32/16 | R |
| Ceftriaxone | >32 | R |
| Cefuroxime | >32 | R |
| Erythromycin | >128 | R |
| Clarithromycin | >32 | R |
| Tetracycline | 16 | R |
| Tigecycline | ≤0.03 | S |
| Levofloxacin | 32 | R |
| Moxifloxacin | 4 | R |
| Gemifloxacin | 1 | R |
| Ciprofloxacin | >32 | R |
| Clindamycin | >32 | R |
| Trimethoprim-sulfamethoxazole | >32/608 | R |
| Meropenem | 16 | R |
| Imipenem | 4 | R |
| Linezolid | 1 | S |
| Vancomycin | 0.25 | S |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download