Abstract
Since severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was noted to cause coronavirus disease 2019 (COVID-19) in 2019, there have been many trials to develop vaccines against the virus. Messenger ribonucleic acid (mRNA) vaccine as a type of the vaccine has been developed and commercialized rapidly, but there was not enough time to verify the long-term safety. An 82-year-old female patient was admitted to the emergency room with dyspnea accompanied by stridor three days after the 3rd COVID-19 mRNA vaccination (Comirnaty, Pfizer-BioNTech, USA). The patient was diagnosed with bilateral vocal fold paralysis (VFP) by laryngoscope. Respiratory distress was improved after the intubation and tracheostomy in sequence. The brain, chest, and neck imaging tests, serological tests, cardiological analysis, and immunological tests were performed to evaluate the cause of bilateral VFP. However, no definite cause was found except for the precedent vaccination. Because bilateral VFP can lead to a fatal condition, a quick evaluation is necessary in consideration of VFP when dyspnea with stridor occurs after vaccination.
Graphical Abstract
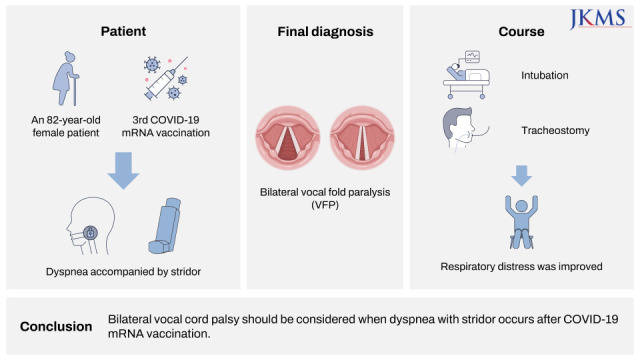
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a single-stranded ribonucleic acid virus which causes coronavirus disease 2019 (COVID-19). For the past two years since the World Health Organization (WHO) declared the COVID-19 pandemic in March 2020, we have been living in the era of the COVID-19 pandemic. Many countries in the world have struggled to overcome the pandemic by vaccine development. The common vaccines contain weakened pathogens (live attenuated vaccine), inactivated pathogens, or parts of the pathogens (non-live vaccine).1 Due to the time taken in manufacturing in these platforms, a completely new method for mRNA vaccines has been developed. It has been studied since the early 21st century against the other pathogens like ebola, zika, rabies, etc.2 mRNA-based vaccines are known to be easier to be developed and be able to produced more quickly than the previous vaccine platforms.3 mRNA vaccines contain 2 components of one RNA encoding the replicase and the other RNA encoding the antigen.4 BNT162b2 vaccine (Comirnaty, Pfizer-BioNTech, USA) has the RNA encoding viral spike glycoprotein of SARS-CoV-2, and its dose is 30 micrograms.5 The United States Food and Drug Administration (FDA) have approved Emergency Use Authorization (EUA) of BNT162b2 vaccine in December 2020 and granted Biological License Application in August 2021. The safety of BNT162b2 vaccine has been proved by several studies, but controversies remain. We report a rare case of bilateral vocal fold paralysis (VFP) after COVID-19 mRNA vaccination, which may be life-threatening.
An 82-year-old female was admitted to the emergency room with dyspnea. The patient had no known other underlying medical diseases except hypertension. She underwent vertebroplasty with local anesthesia for compression fractures in 11th and 12th thoracic vertebrae before 7 days and had been recuperating in a nursing hospital. The COVID-19 polymerase chain reaction was negative before the procedure. There were no upper respiratory symptoms before and after the procedure, and no other postoperative complications were observed. Two days after the procedure, she got the 3rd BNT162b2 COVID-19 mRNA vaccination (Comirnaty, Pfizer-BioNTech, USA) because of the policy about health regulation.
Two days after vaccination, she complained of mild dyspnea and the doctor kept using the antibiotics that had been used after the procedure. The next day, her symptom aggravated with inspiratory stridor, so the patient had to be taken to the emergency medical center. The mental status was alert and respiratory rate increased with inspiratory stridor. The laboratory findings including white blood cell count, segmented neutrophils, hemoglobin, platelets, and pro-calcitonin were within normal ranges; erythrocyte sedimentation rate (ESR) (25 mm/hr [normal range 0–20]) and C-reactive protein (CRP) (8.85 mg/dL [normal range 0.0–5.0]) were slightly elevated. The arterial blood gas analysis (ABGA) showed the oxygen saturation of 95.7%, partial pressure of carbon dioxide (pCO2) of 40.0 mmHg, hydrogen carbonate (HCO3) of 27.5 mmol/L, and power of hydrogen (pH) of 7.45. The chest X-ray revealed cardiomegaly and mild bilateral pleural effusion without any sign of pneumonia. Atrial fibrillation was observed on electrocardiogram and cardiac markers were mildly elevated: Pro-B-type natriuretic peptide, 5,415 pg/mL (normal range < 300); troponin T, 29.3 ng/L (normal range < 14); creatine kinase myocardial band, 24.1 ng/mL (normal range 0–3.6). Echocardiogram was performed to rule out cardiac origin diseases such as chronic heart failure. Left atrium chamber size was slightly increased, but ejection fraction was approximately 65–70%, which was within normal range without regional wall motion abnormality. The other findings in echocardiogram were normal. The upper airway was examined by a portable flexible fiberoptic laryngoscope. Bilateral VFP in median position was observed and the airway was open only in the posterior 10–20%. Finally, we confirmed that respiratory distress occurred by acute bilateral VFP. After intubation, a ventilator was applied immediately and tracheostomy was done in the next day. As the serial results of ABGA improved and the patient appealed respiratory comfort, the ventilator with oxygen supplement was removed on the hospital day 2.
To check the status of bilateral vocal folds, stroboscopy was done one day after the tracheostomy (Supplementary Video 1). Median position bilateral VFP was still observed with loosened vocal fold mucosa due to the paralysis of posterior cricoarytenoid muscle. Saliva pooling was also seen because of the dysfunction of cricopharyngeal muscle. Although obvious dysphagia was not observed, because vocal closure was impossible and saliva pooling was observed, a nasogastric tube was inserted to protect the airway from aspiration. Due to slightly elevated ESR, CRP and invasive tracheostomy, piperacillin and tazobactam started. Steroid therapy or anti-viral therapy was considered, but we concluded that there was little benefit to this patient because of chronic heart failure and change of creatinine, aspartate aminotransferase, and alanine aminotransferase.
Further studies to find out the cause of bilateral VFP were performed. In physical examination, all cranial nerves were intact except for the vagus nerve. Especially, olfactory function and extraocular movement were normal. In computed tomography (CT), there were no lesions in the thyroid, trachea, aortic arch, lung, and whole tract vagus nerve runs through. Brain magnetic resonance imaging (MRI) showed no specific findings. Autoimmune serology results (anti ds-DNA Ab, anti-nuclear Ab, cryoglobulin, rheumatoid factor, complement C3, complement C4, immunoglobulin G, and immunoglobulin M) were in the normal range. Fourteen days after the tracheostomy, electromyography was done and the result was compatible with bilateral VFP. After 2 months, VFP still remained, but saliva pooling was improved (Supplementary Video 2). The patient performed well in the modified barium swallowing test, so she started oral feeding. LASER posterior cordotomy is planned if bilateral VFP persists longer.
Bilateral VFP after vaccination is very rare symptom. Although the safety of BNT162b2 vaccine has been proved by several studies, controversies remain. One of the most well-known side effect is myocarditis in adolescents.6 Facial palsy after vaccination has been also reported. However, not in case reports but in review articles, facial palsy is rare and its relationship with vaccination is not clear.27 Similarly, VFP has also been reported and there are 2 studies about VFP associated with COVID-19 vaccination.89 Lehrer et al.9 have reported 2 cases of unilateral VFP after BNT162b2 vaccination. Hamdi et al.8 have documented 19 cases using The United States Vaccine Adverse Event Reporting System (VAERS) database. They reported that the mean lag time between vaccination and the symptom onset was about 12 days, and the mean time for diagnosis by laryngoscopy was 37 days. They also reported that 2 patients had bilateral VFP, but did not describe the course of VFP and whether a tracheostomy was done.
Although bilateral VFP is life-threatening disease, this is not easy to be diagnosed in early stage. The best diagnostic tool of bilateral VFP is laryngoscope, but we must consider bilateral VFP even if only some symptoms are present, such as pattern of breath, movement of extra-pulmonary muscles, and especially characteristic stridor. In this case, the 82-year-old female had dyspnea with inspiratory stridor only three days after vaccination. The cause of VFP is various.10 Trauma including thyroid, cervical spine, thoracic surgery, and endotracheal intubation can cause VFP. In addition, the injury of central or peripheral nerve system, cerebrovascular disease, tumor, and specific neuropathy can result in VFP. In this patient, the fractured 11th and 12th thoracic vertebrae are not related with vocal fold mobility. Brain MRI and CT scan from skull base to chest showed no lesion along the pathway of vagus nerve, particularly, the recurrent laryngeal nerve. Polyneuropathy induced by Guillain-Barre syndrome (GBS) and rheumatic diseases such as systemic lupus erythematosus or rheumatoid arthritis can also cause VFP. However, the patient had no symptoms other than VFP and dysphagia, and specific antibody tests to discriminate systemic autoimmune disease were negative. Of course, idiopathic VFP can also occur in old age, but in this case, it is highly likely that it was caused by vaccination in terms of close temporal relation.
There is a report about the relationship between common vaccinations and VFP.11 Talmor et al.11 reviewed VAERS database from July 1990 to October 2019 and reported 22 vocal fold immobility cases following vaccinations. Vaccinations including influenza, hepatitis B, herpes zoster, and pneumococcus have caused VFP. There were 4 cases of bilateral vocal fold immobility and the mean latent period was 6.3 days. They referred VFP to post-vaccination neuropathy.
There have been many reports of neurological symptoms in patients infected with COVID-19. Anosmia and ageusia have been reported in 59.45% of COVID-19 patients as the most common neurologic disorders.1213 Peripheral nerve disorders like facial palsy, sudden sensory neural hearing loss, and GBS have also been reported.14 It is known that SARS-CoV-2 uses angiotensin-converting enzyme 2 (ACE2) as a receptor to enter human host cells causing severe COVID-19 syndromes.14 ACE2 is expressed highly in the lung, intestines, esophagus, cardiomyocytes, and neurons.15 Both peripheral and central nerves can be targets of SARS-CoV-2. The SARS-CoV-2 spike protein has an important role in initiating the interaction between the virus and host cell surface receptor, assisting viral entry into the host cell by the fusion of viral and host cell membranes. Since the mRNA vaccine contains mRNA that encodes the viral spike glycoprotein of SARS-CoV-2, it may induce peripheral nerve disorders by the same fusion mechanism between the spike protein and neural membrane as in SARS-CoV-2 infection. Although this hypothesis needs to be evidenced by further research, our short-latency VFP suggests more probably the fusion mechanism rather than an immune mechanism.
The present case is thought to be the first case of bilateral VFP that occurred only three days after mRNA vaccination and was treated with a tracheostomy. It has been two months since the tracheostomy, but the patient still has bilateral VFP. VFP has not so far been established as one of the known complications of COVID-19 vaccination. Through this case, we consider the possibility that VFP can occur as a side effect of COVID-19 vaccination. As the mRNA vaccine platform is emerging on a large scale for the first time, it may be important to report any suspected complications. More studies are needed to prove the relationship between VFP and COVID-19 mRNA vaccination.
References
1. Vetter V, Denizer G, Friedland LR, Krishnan J, Shapiro M. Understanding modern-day vaccines: what you need to know. Ann Med. 2018; 50(2):110–120. PMID: 29172780.

2. Anand P, Stahel VP. Review the safety of Covid-19 mRNA vaccines: a review. Patient Saf Surg. 2021; 15(1):20. PMID: 33933145.
3. Shang W, Yang Y, Rao Y, Rao X. The outbreak of SARS-CoV-2 pneumonia calls for viral vaccines. NPJ Vaccines. 2020; 5(1):18. PMID: 32194995.

4. Wang Y, Zhang Z, Luo J, Han X, Wei Y, Wei X. mRNA vaccine: a potential therapeutic strategy. Mol Cancer. 2021; 20(1):33. PMID: 33593376.

5. Kadali RA, Janagama R, Yedlapati SH, Kanike N, Gajula V, Madathala RR, et al. Side effects of messenger RNA vaccines and prior history of COVID-19, a cross-sectional study. Am J Infect Control. 2022; 50(1):8–14. PMID: 34718069.

6. Gargano JW, Wallace M, Hadler SC, Langley G, Su JR, Oster ME, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the advisory committee on immunization practices - United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021; 70(27):977–982. PMID: 34237049.

7. Ahsanuddin S, Nasser W, Roy SC, Povolotskiy R, Paskhover B. Facial paralysis and vaccinations: a vaccine adverse event reporting system review. Fam Pract. 2022; 39(1):80–84. PMID: 34184737.

8. Hamdi OA, Jonas RH, Daniero JJ. Vocal fold paralysis following COVID-19 vaccination: query of VAERS database. J Voice. Forthcoming. 2022; DOI: 10.1016/j.jvoice.2022.01.016.

9. Lehrer E, Jubés S, Casanova-Mollà J. Vocal fold palsy after vaccination against SARS-CoV-2. Med Clin (Barc). Forthcoming. 2022; DOI: 10.1016/j.medcli.2021.12.013.

10. Stager SV. Vocal fold paresis: etiology, clinical diagnosis and clinical management. Curr Opin Otolaryngol Head Neck Surg. 2014; 22(6):444–449. PMID: 25254404.
11. Talmor G, Nguyen B, Din-Lovinescu C, Paskhover B, Kaye R. Vocal fold immobility following vaccination. Ann Otol Rhinol Laryngol. 2021; 130(6):609–613. PMID: 33063519.

12. Vaira LA, Salzano G, Deiana G, De Riu G. Anosmia and ageusia: common findings in COVID-19 patients. Laryngoscope. 2020; 130(7):1787. PMID: 32237238.
13. Butowt R, von Bartheld CS. Anosmia in COVID-19: underlying mechanisms and assessment of an olfactory route to brain infection. Neuroscientist. 2021; 27(6):582–603. PMID: 32914699.

14. Nepal G, Rehrig JH, Shrestha GS, Shing YK, Yadav JK, Ojha R, et al. Neurological manifestations of COVID-19: a systematic review. Crit Care. 2020; 24(1):421. PMID: 32660520.

15. Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020; 11(7):995–998. PMID: 32167747.





 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download