1. Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011; 34:274–285. PMID:
21623852.

2. Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004; 40:1387–1395. PMID:
15565570.

3. Kim HC, Choi KS, Jang YH, Shin HW, Kim DJ. Normal serum aminotransferase levels and the metabolic syndrome: Korean National Health and Nutrition Examination Surveys. Yonsei Med J. 2006; 47:542–550. PMID:
16941745.

4. Kojima S, Watanabe N, Numata M, Ogawa T, Matsuzaki S. Increase in the prevalence of fatty liver in Japan over the past 12 years: analysis of clinical background. J Gastroenterol. 2003; 38:954–961. PMID:
14614602.

5. Park SH, Jeon WK, Kim SH, Kim HJ, Park DI, Cho YK, et al. Prevalence and risk factors of non-alcoholic fatty liver disease among Korean adults. J Gastroenterol Hepatol. 2006; 21(1 Pt 1):138–143. PMID:
16706825.

6. Jeong EH, Jun DW, Cho YK, Choe YG, Ryu S, Lee SM, et al. Regional prevalence of non-alcoholic fatty liver disease in Seoul and Gyeonggi-do, Korea. Clin Mol Hepatol. 2013; 19:266–272. PMID:
24133664.

7. Younossi Z, Tacke F, Arrese M, Chander Sharma B, Mostafa I, Bugianesi E, et al. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology. 2019; 69:2672–2682. PMID:
30179269.

8. Younossi ZM. Non-alcoholic fatty liver disease: a global public health perspective. J Hepatol. 2019; 70:531–544. PMID:
30414863.
9. Palmer M, Schaffner F. Effect of weight reduction on hepatic abnormalities in overweight patients. Gastroenterology. 1990; 99:1408–1413. PMID:
2210247.

10. Park HS, Kim MW, Shin ES. Effect of weight control on hepatic abnormalities in obese patients with fatty liver. J Korean Med Sci. 1995; 10:414–421. PMID:
8924225.

11. Nickel F, Schmidt L, Bruckner T, Billeter AT, Kenngott HG, Müller-Stich BP, et al. Gastrointestinal quality of life improves significantly after sleeve gastrectomy and Roux-en-Y gastric bypass: a prospective cross-sectional study within a 2-year follow-up. Obes Surg. 2017; 27:1292–1297. PMID:
27878423.

12. Nickel F, Schmidt L, Bruckner T, Büchler MW, Müller-Stich BP, Fischer L. Influence of bariatric surgery on quality of life, body image, and general self-efficacy within 6 and 24 months: a prospective cohort study. Surg Obes Relat Dis. 2017; 13:313–319. PMID:
28029597.

13. Chon YE, Jung KS, Kim SU, Park JY, Park YN, Kim DY, et al. Controlled attenuation parameter (CAP) for detection of hepatic steatosis in patients with chronic liver diseases: a prospective study of a native Korean population. Liver Int. 2014; 34:102–109. PMID:
24028214.

14. Chon YE, Jung KS, Kim KJ, Joo DJ, Kim BK, Park JY, et al. Normal controlled attenuation parameter values: a prospective study of healthy subjects undergoing health checkups and liver donors in Korea. Dig Dis Sci. 2015; 60:234–242. PMID:
25118979.

15. Lee JH, Kim D, Kim HJ, Lee CH, Yang JI, Kim W, et al. Hepatic steatosis index: a simple screening tool ref lecting nonalcoholic fatty liver disease. Dig Liver Dis. 2010; 42:503–508. PMID:
19766548.

16. Chang JW, Lee HW, Kim BK, Park JY, Kim DY, Ahn SH, et al. Hepatic steatosis index in the detection of fatty liver in patients with chronic hepatitis b receiving antiviral therapy. Gut Liver. 2021; 15:117–127. PMID:
32066210.

17. Botros M, Sikaris KA. The de ritis ratio: the test of time. Clin Biochem Rev. 2013; 34:117–130. PMID:
24353357.
18. Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003; 38:518–526. PMID:
12883497.

19. Hall P, Cash J. What is the real function of the liver ‘function’ tests? Ulster Med J. 2012; 81:30–36. PMID:
23536736.
20. Loaeza-del-Castillo A, Paz-Pineda F, Oviedo-Cárdenas E, Sánchez-Avila F, Vargas-Vorácková F. AST to platelet ratio index (APRI) for the noninvasive evaluation of liver fibrosis. Ann Hepatol. 2008; 7:350–357. PMID:
19034235.

21. Gordon SC, Kachru N, Parker E, Korrer S, Ozbay AB, Wong RJ. Health care use and costs among patients with nonalcoholic steatohepatitis with advanced fibrosis using the fibrosis-4 score. Hepatol Commun. 2020; 4:998–1011. PMID:
32626832.

22. Kang SH, Lee HW, Yoo JJ, Cho Y, Kim SU, Lee TH, et al. KASL clinical practice guidelines: Management of nonalcoholic fatty liver disease. Clin Mol Hepatol. 2021; 27:363–401. PMID:
34154309.

23. Nascimento TM, Alves-Júnior A, Nunes MA, de Freitas TR, da Silva MA, Alves MR. Comparison of hepatic profile in pre and postoperative of bariatric surgery: private vs public network. Arq Bras Cir Dig. 2015; 28:274–277. PMID:
26734800.

24. Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007; 46:32–36. PMID:
17567829.

25. Jun DW, Kim SG, Park SH, Jin SY, Lee JS, Lee JW, et al. External validation of the non-alcoholic fatty liver disease fibrosis score for assessing advanced fibrosis in Korean patients. J Gastroenterol Hepatol. 2017; 32:1094–1099. PMID:
27859583.

26. Hafeez S, Ahmed MH. Bariatric surgery as potential treatment for nonalcoholic fatty liver disease: a future treatment by choice or by chance? J Obes. 2013; 2013:839275. PMID:
23431426.

27. Mummadi RR, Kasturi KS, Chennareddygari S, Sood GK. Effect of bariatric surgery on nonalcoholic fatty liver disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2008; 6:1396–1402. PMID:
18986848.

28. Lee Y, Doumouras AG, Yu J, Brar K, Banfield L, Gmora S, et al. Complete resolution of nonalcoholic fatty liver disease after bariatric surgery: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2019; 17:1040–1060. PMID:
30326299.

29. Naveau S, Lamouri K, Pourcher G, Njiké-Nakseu M, Ferretti S, Courie R, et al. The diagnostic accuracy of transient elastography for the diagnosis of liver fibrosis in bariatric surgery candidates with suspected NAFLD. Obes Surg. 2014; 24:1693–1701. PMID:
24841950.

30. Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012; 55:2005–2023. PMID:
22488764.

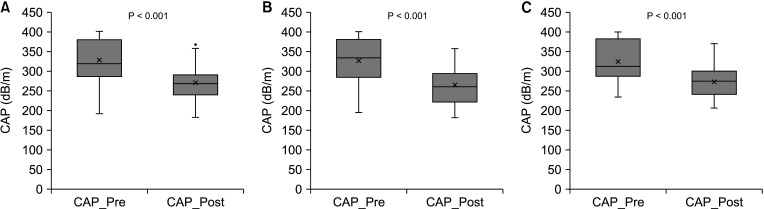
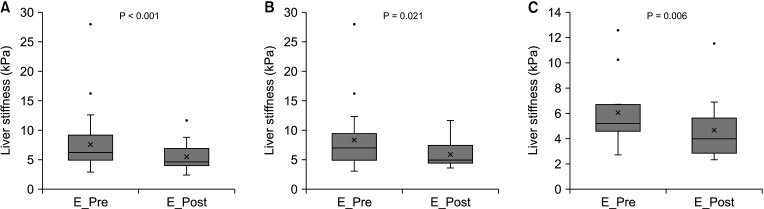
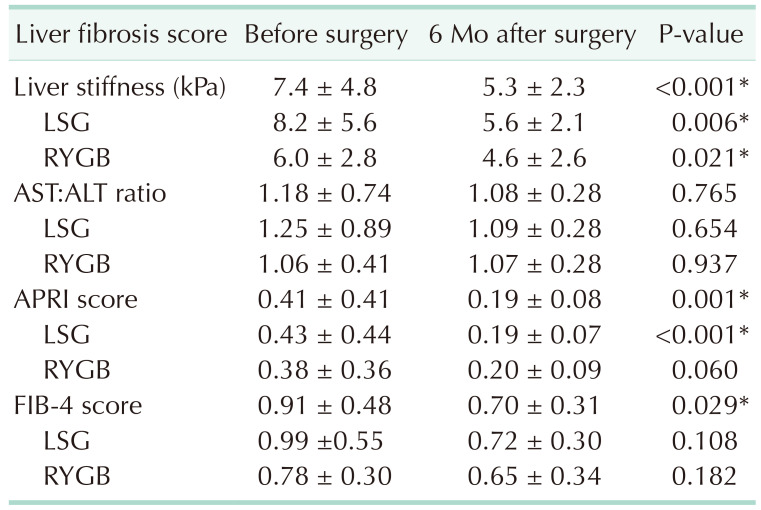
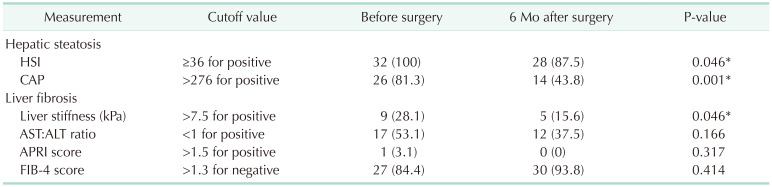




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download