Abstract
Purpose
Postoperative adhesions after thyroidectomy adversely affect patients’ quality of life. This study assessed the anti-adhesive effect and safety of thermosensitive sol-gel agents for patients undergoing thyroidectomy.
Methods
A double-blind parallel-group randomized clinical trial involving patients aged 20–70 years undergoing thyroidectomy for thyroid nodules was conducted. From August 2017 to April 2020, 90 patients were randomly assigned to the experimental (n = 45, thermosensitive sol-gel agent applied to the surgical site) and control (n = 45, no treatment) groups in a 1:1 ratio. All patients were assessed using a questionnaire for swallowing difficulty, wrinkle problems, and inflammation at 2 weeks, 3 months, and 6 months after thyroid surgery. For reoperated patients, the degree of adhesion was evaluated according to the adhesion-evaluation score system (range, 0–4).
Results
During the follow-up period of 6.50 ± 1.38 months, the swallowing difficulty, wrinkle problem, and inflammation were improved in both groups. However, there was no statistically significant difference between the control and experimental groups related to swallowing difficulty, wrinkle problems, and inflammation. Two patients in the control group and 1 in the experimental group who underwent reoperation had an adhesion-evaluation score of 3 points. There were no adverse effects or allergic reactions.
According to the Korea Central Cancer Registry report, the estimated incidence of thyroid cancer in Korea was 27,747, the third-highest after lung cancer and stomach cancer [1]. The estimated age-standardized incidence of thyroid cancer was 36.4 out of 100,000, which was the highest incidence [1]. More than 80%–90% of thyroid cancers are well-differentiated cancers such as papillary thyroid cancer (PTC) and follicular thyroid cancer (FTC), which are known to have a good prognosis [23]. It has been reported that patients with PTC have a 20-year survival rate of >90%, and FTC has a 10-year survival rate of 80% [23]. It is important for thyroid cancer patients to maintain their long-term quality of life [4].
Surgical resection is the primary treatment for thyroid cancer. Thyroidectomy can cause various complications, such as hypoparathyroidism and vocal cord paralysis, postoperative bleeding, chyle leakage, and postoperative adhesions. Although many studies have been performed on hypoparathyroidism or vocal cord paralysis after thyroid surgery, only a few studies have investigated neck adhesions after surgery [5]. Postoperative adhesions are natural postoperative reactions that occur in the tissues surrounding the surgical site. Due to adhesions, patients complain of dysphagia, persistent abnormal sensations or pain, movement disorders, and abnormal skin wrinkles [67]. Several studies related to the use of anti-adhesion agents to improve the quality of life of patients by preventing adhesions after thyroid surgery have been conducted, but they did not show any significant improvement in patients’ adhesion-related symptoms [568].
Mediclore (CGBio Co., Ltd., Seongnam, Korea) is a thermosensitive sol-gel anti-adhesion solution mixed with a highly biocompatible and water-soluble polymer formulation capable of sol-gel transition. Mediclore exists as a sol (liquid) at room temperature; it becomes a high-viscosity gel when it reaches body temperature after application inside the human body, and it is expected to have an excellent anti-adhesive effect compared to conventional anti-adhesion agents [9]. Several studies have reported on the anti-adhesive effect of Mediclore for the following surgeries: (1) intranasal dacryocystectomy; (2) transurethral resection for benign prostatic hyperplasia; (3) rotator cuff repair; and (4) abdominal gynecological surgery [9101112]. However, no studies analyzing the effect of Mediclore on thyroid surgery have not been conducted. Therefore, we conducted a prospective randomized controlled clinical trial (RCT) to confirm the anti-adhesive effect and safety of Mediclore in thyroidectomy using a double-blind method. We present the following article in accordance with the CONSORT (Consolidated Standards of Reporting Trials) reporting guidelines (http://www.consort-statement.org/).
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The trial was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Seoul National University Hospital (No. D-1611-088-809) and informed consent was taken from all individual participants.
This study was a parallel-designed prospective single-center RCT. All participants who provided informed consent to participate in the present study were allocated to 2 groups in a 1:1 ratio; the Mediclore group and the control group. Between August 2017 and April 2020, 90 patients who underwent thyroid surgery for thyroid nodules were enrolled in this study.
This RCT was registered on “ClinicalTrials.gov” (identifier, NCT03293186; https://clinicaltrials.gov/ct2/show/NCT03293186) on September 26, 2017.
The inclusion criteria were as follows: (1) patients aged between 20 years and 70 years; (2) patients who provided written informed consent; (3) patients who were scheduled to undergo thyroid surgery; (4) no evidence of tumor invasion to adjacent tissues or distant metastasis for thyroid cancer patients; (5) patients whose vocal cord evaluation before thyroid surgery showed normal movement; and (6) patients with no abnormal findings of preoperative laboratory tests.
The following patients were excluded: (1) those who were confirmed as inappropriate candidates for this study by the researchers; (2) those who took aspirin or antiplatelet agents at ≤7 days before the surgery; (3) those who had uncontrolled hypertension, diabetes mellitus, chronic renal disease, or coagulopathy; (4) those who had cardiovascular disease, such as angina pectoris, heart failure, myocardial infarct, coronary artery disease, stroke or transient ischemic attack; (5) those who had past history of drug abuse or alcohol abuse; (6) those who had history of esophagus or airway disease; (7) those who had keloid or hypertrophic scar; (8) those who participated in another clinical trial within 1 month; (9) patients who planned to perform modified radical neck dissection due to the evidence of lateral compartment lymph node metastasis; (10) patients suspected to have thyroiditis, such as Graves’ disease or Hashimoto thyroiditis; (11) patients who had clinical cervical adhesion before surgery because of previous history of radiation therapy or surgery at neck; (12) patients with a history of allergic reaction to drugs, especially poloxamer, gelatin, or chitosan; or (13) female participants who were pregnant or breastfeeding.
All the participants underwent conventional open thyroid surgery. All procedures performed during surgery were the same for the Mediclore and control groups, except the closing of the operative field. For the Mediclore group, the wound was closed using Mediclore in the thyroid operative bed and subplatysmal flap area; for the control group, the wound was closed without anti-adhesive agents. The surgeons who performed the closure procedure were blinded to the treatment allocations until they got to the operating room.
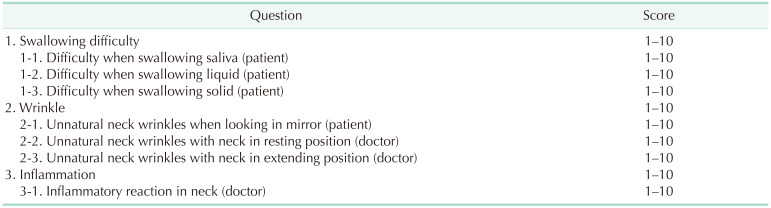
The primary endpoint of this study was the severity of postoperative adhesions based on questionnaires used in a previous study conducted at the same institution [81314]. The questions in the questionnaire had numeric scores ranging from 1 (no discomfort) to 10 (worst discomfort) points. They included swallowing difficulty, naturalness of the neck wrinkles, and inflammatory reactions. The detailed questionnaire questions are presented in Table 1. The questionnaire surveys were performed 3 times for patients and researchers at the outpatient clinic 2 weeks, 3 months, and 6 months postoperatively. The scores of the items for each question group (swallowing difficulty, naturalness of the neck wrinkles, and inflammatory reaction) at each time point (2 weeks, 3 months, and 6 months after surgery) were summed for comparison of the 2 groups.
The secondary endpoints were the severity of adhesion found during the completion of thyroidectomy and the safety of Mediclore. The researchers evaluated the efficacy of Mediclore by assessing the degree of postoperative adhesion during the second surgery according to the adhesion-evaluation score system (0, no adhesion; 1, mild adhesion and easy dissection; 2, moderate adhesion with moderately difficult dissection causing mild injury to adjacent organs; 3, severe fibrotic adhesion with difficult dissection; and 4, severe fibrotic adhesion with difficulty in identifying anatomical/surgical planes).
The safety of Mediclore was investigated by evaluating the incidence of adverse events, such as allergic reactions, inflammatory reactions, or other wound problems.
Previous studies have been conducted on 86, 162, and 76 patients to evaluate the efficacy of the anti-adhesion agent after thyroid surgery, but the efficacy was not proven [81314]. It was impossible to count the number of samples because there were no previous studies demonstrating the efficacy of the anti-adhesion agent. This study did not follow accurate statistical procedures in the process of calculating the number of study participants. Therefore, we planned to perform this RCT on 80 participants. Considering a dropout rate of 10%, 90 participants were enrolled in this RCT.
A simple random assignment was performed using a random assignment function created in R software ver. 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria). As the anti-adhesive agent had to be applied by the operator, the researchers participating in the surgery (SK, KEL) were aware of the patient’s assignment. However, the remaining researchers remained double-blinded until the end of the study, and they conducted the survey. In addition, to minimize the operator’s bias, a randomized group was opened to the researchers in the operation during the closure of the wound after thyroid surgery.
Statistical analysis was performed using R software ver. 4.0.2. Continuous variables, such as the operating time and the blood loss, were analyzed using Student t-test for normal distribution variables and the Mann-Whitney test for non-normally distributed variables. The categorical variables were analyzed using the chi-square test and Fisher exact test for >20% of the variables with expected frequencies of <5. The questionnaire scores of the Mediclore and control groups at each time point were compared using a repeated-measures analysis of variance test. Statistical significance was set at a P-value of <0.05.
Of the 90 patients enrolled in this RCT, 81 patients were included in the analysis. Nine patients were excluded from the study for the following reasons: (1) withdrawal of consent (n = 1, control group); (2) loss of follow-up (n = 1, Mediclore group); and (3) protocol violations (n = 7; 3 and 4 individuals in the control and Mediclore groups, respectively). Therefore, data from 81 patients (41 in the control group and 40 in the Mediclore group) were used for analysis (Fig. 1, Table 2).
The total follow-up duration was 6.5 ± 1.38 months (6.5 ± 1.6 months in the control group and 6.5 ± 1.2 months in the Mediclore group; P = 0.828). Age, sex, and body mass index were not significantly different between the 2 groups (P = 0.713, P = 0.280, and P = 0.503, respectively). The number of patients who underwent total thyroidectomy, the extent of central compartment lymph node dissection (CND), and the number of patients who underwent frozen biopsy were similar in the 2 groups (P = 0.440, P = 0.932, and P = 0.923, respectively). The number of patients confirmed to have malignancy based on the final pathological studies was 34 (82.9%) in the control group and 35 (87.5%) in the Mediclore group (P = 0.800). The longest diameter of the tumor was similar for the 2 groups (control group, 1.2 ± 0.9 cm vs. Mediclore group, 1.2 ± 1.2 cm; P = 0.855).
The duration of surgery was significantly longer in the Mediclore (75.5 ± 22.6 minutes) than in the control group (65.4 ± 17.0 minutes, P = 0.027). The difference in the duration of surgery related to the presence or absence of frozen biopsy was not statistically significant for the control group (P = 0.528), but it was statistically significant for the Mediclore group (P < 0.05) (Supplementary Table 1). There was no difference in the duration of drainage for the 2 groups, but the amount of drainage was higher in the Mediclore group than in the control group (control group, 60.8 ± 25.6 mL vs. Mediclore group, 89.1 ± 42.1 mL; P = 0.001).
The number of patients diagnosed with transient hypoparathyroidism was 1 in each group. Postoperative vocal cord palsy (VCP) was confirmed in 4 patients (10.0%) in the Mediclore group: 3 patients (7.5%) with transient VCP, and 1 patient (2.5%) with permanent VCP. There were no cases of surgical site infection, postoperative bleeding, or allergic reactions in either group.
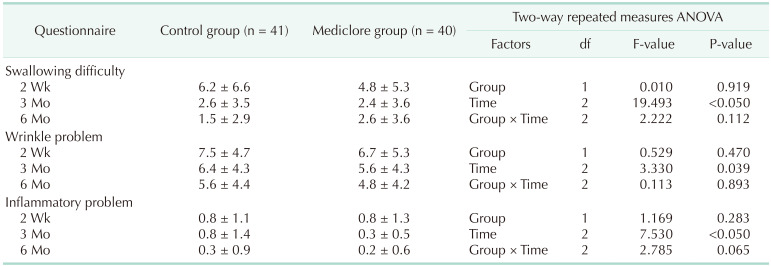
There was no difference in the degree of difficulty with swallowing between the 2 groups based on the questionnaire survey of the postoperative adhesions at 2 weeks postoperatively; the control group had 6.2 ± 6.6 and the Mediclore group had 4.8 ± 5.3 (P = 0.349) (Fig. 2, Table 3). There was no difference between the 2 groups based on the results of the questionnaire survey, even when the difficulty in swallowing was divided based on its association with saliva, liquids, and solids (P = 0.887, P = 0.246, and P = 0.151, respectively). Similar results were found between the control (7.5 ± 4.7) and Mediclore groups (6.7 ± 5.3) for the questionnaire survey of wrinkle problems at 2 weeks postoperatively (P = 0.271) (Fig. 3, Table 3). There were no differences in the outcomes between the 2 groups when wrinkle problems were assessed using the questionnaire in 3 ways: (1) subjective patient evaluation, (2) physical examination in resting neck position, and (3) physical examination in neck extension position (P = 0.442, P = 0.439, and P = 0.140, respectively).
Similar results of the questionnaire survey for the evaluation of swallowing difficulty at 3 months postoperatively were also observed (2.6 ± 3.5 for the control group vs. 2.4 ± 3.6 for the Mediclore group, P = 0.584) (Fig. 2, Table 3). Dysphagia when swallowing saliva, liquids, or solid substances was similar for the 2 groups (P = 0.704, P = 0.693, and P = 0.983, respectively). The questionnaire scores for the wrinkle problems assessed by the participants and the researchers for the 2 postures (resting and extending the neck) did not differ between the 2 groups (P = 0.374, P = 0.258, and P = 0.896, respectively) (Fig. 3, Table 3).
The adhesion-related efficacy questionnaire survey conducted at the outpatient clinic 6 months postoperatively showed swallowing difficulty scores of 1.5 ± 2.9 for the control group and 2.6 ± 3.6 for Mediclore group (P = 0.262) (Fig. 2, Table 3). There was no difference between the 2 groups when swallowing difficulty was evaluated by distinguishing the swallowing of saliva, liquids, and solids at 6 months postoperatively (P = 0.278, P = 0.140, and P = 0.740, respectively). At 6 months postoperatively, there was no difference between the 2 groups related to the participants’ evaluation of the patient’s neck wrinkles, the doctor’s examination at rest, and the doctor’s examination at the time of neck extension (P = 0.782, P = 0.933, and P = 0.887, respectively) (Fig. 3, Table 3).
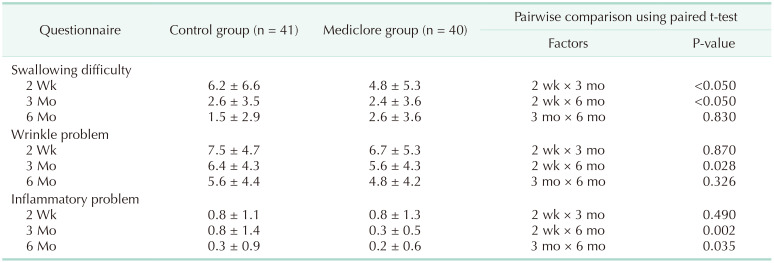
Table 4 shows the results of the analysis by time and group of questionnaires conducted 3 times at 2 weeks, 3 months, and 6 months postoperatively. For the swallowing difficulty, there was a significant difference in the temporal change pattern at 2 weeks, 3 months, and 6 months postoperatively (P < 0.05), but there was no difference between the control and Mediclore groups (P = 0.919). For the wrinkle-related questionnaire items, there was a significant difference in the changing pattern over time (P = 0.039), and there was no difference between the 2 groups (P = 0.47).
The analysis of the time interval related to dysphagia showed that the evaluation score at 2 weeks was significantly higher than the evaluation scores at 3 and 6 months (P < 0.05 and P < 0.05, respectively), and there was no difference between the questionnaire scores at 3 and 6 months (P = 0.83) (Table 5). The analysis of the time-related differences in the wrinkles showed that the questionnaire evaluation score was significantly higher in the 2nd week than in the 6th month (P = 0.028), and there was no significant difference during the 3rd month relative to the scores at 2 weeks and 6 months (P = 0.870 and P = 0.326, respectively).
Three participants underwent completion thyroidectomy; 2 patients in the control group and 1 in the Mediclore group (Supplementary Table 2). Statistical analysis of the secondary outcomes related to the severity of adhesion during completion thyroidectomy was impossible because there were 3 cases. The first patient in the control group underwent right lobectomy for a 4.1-cm thyroid nodule. After the final pathological results indicated minimally invasive FTC measuring 4.1 cm, the patient underwent completion thyroidectomy 115 days after the first surgery. During the completion of thyroidectomy, the adhesion-evaluation rating score of the researcher was 3. The other patient in the control group who underwent left lobectomy with left CND was confirmed to have PTC measuring 2.1 cm and central lymph node metastasis (CLNM). The patient had an adhesion-evaluation score of 3 during the completion of the right lobectomy with right CND 138 days after the first surgery. PTC measuring 0.5 cm and multiple CLNMs by right lobectomy with right CND was confirmed for 1 patient in the Mediclore group. After 119 days, left lobectomy with left CND was performed with an adhesion-evaluation score of 3.
There were no cases of adverse effects or allergic reactions to Mediclore (Table 3). The questionnaire for evaluating inflammatory reactions at 2 weeks, 3 months, and 6 months postoperatively showed similar results for the 2 groups (P = 0.629, P = 0.311, and P = 0.821, respectively) (Fig. 4, Table 3). Regarding inflammation, there were significant differences in the time-dependent change patterns at 2 weeks, 3 months, and 6 months postoperatively (P < 0.05). The evaluation score at 6 months was significantly lower than the evaluation scores at 2 weeks and 3 months (P = 0.002 and P = 0.035, respectively), and there was no difference between the scores at 2 weeks and 3 months (P = 0.490) (Table 5). However, there was no difference between the control and Mediclore groups (P = 0.283) (Table 4).
In this RCT, Mediclore was safely used without adverse effects, but the improvement in patient adhesion was not confirmed. The advantage of this study was that postoperative adhesion was evaluated based on subjective symptoms and objective examinations over 6 months. To the best of our knowledge, this is the first study to confirm the anti-adhesive effect of Mediclore in thyroid surgery.
Postoperative adhesion refers to a phenomenon, in which tissues or organs of the human body are naturally separated from fibrous tissue but not from each other [15]. Organ damage and epithelial cell surface loss at the surgical site induce an inflammatory response that results in the production of serous exudates and activates the extrinsic pathway of the coagulation cascade, leading to initial fibrosis [16]. The fibrinolytic process, which occurs under normal circumstances, is inhibited due to ischemic or inflammatory conditions after surgery, and fibroblasts proliferate, which worsens adhesion.
Postoperative adhesions can lead to complications and cosmetic problems. After thyroidectomy, the postoperative thyroid surgery the site and adjacent tissues, such as the trachea, esophagus, scapula, and cranial muscle, attach directly to the scar, which can cause difficulty in swallowing and voice changes [1718].
There are 3 strategies for preventing postoperative adhesion: first, a method for minimizing tissue damage and foreign substances through delicate and necessary touch during surgery; second, a method for applying drug treatment based on the adhesion mechanism; and third, a method to place exogenous anti-adhesive barriers to block the contact between the operative bed and surrounding tissues [719]. However, there are no unique products with excellent anti-adhesive effects [781314].
Several studies have been conducted to find substances to prevent adhesions after surgery; however, the effectiveness of these substances has not been proven [781420]. Therefore, it is important to secure a substance that prevents postoperative adhesions after thyroidectomy. This study used an anti-adhesion agent called Mediclore, a temperature-sensitive sol-gel composed of poloxamer, gelatin, and chitosan. Mediclore acts as an external barrier to prevent adhesion until absorbed for approximately 20 days. Anti-adhesive and antibacterial effects of Mediclore have been reported in various studies [91011122122].
In this study, swallowing difficulty and wrinkle problems were the most severe at 2 weeks after thyroid surgery and were improved at 3 months, but there was no statistical difference between the 2 groups. All 3 patients who underwent reoperation showed the same degree of adhesion, so the anti-adhesive effect of Mediclore could not be confirmed (n = 3; 2 and 1 individuals in the control and experimental groups, respectively). However, statistical analysis was impossible because of the few reoperations; hence, studies with a larger sample size are needed.
Similar to several previous studies, this study confirmed that Mediclore could be used safely [9101112]. Inflammatory assessment by questionnaire confirmed the absence of Mediclore-associated inflammatory response. Temporary and permanent recurrent laryngeal nerve palsy occurred more frequently in the Mediclore group. However, the difference was not statistically significant, and these complications were more related to the difficulty of the operation than to the use of Mediclore. In addition, Mediclore was safe to use without any surgical site infection, postoperative bleeding, or allergic reactions.
The duration of surgery in the experimental group (75.5 ± 22.6 minutes) was 10 minutes longer than that of the control group (65.4 ± 17.0 minutes). As it takes <1 minute to inject 5 mL of Mediclore into the surgical site, it can be inferred that the difference in the duration of surgery of 10 minutes between the 2 groups was because the variables acted as confounding variables. Researchers considered that frozen biopsy could be a confounding factor because the frozen biopsy time differed by more than 10 minutes between the experimental and control groups (Supplementary Table 1). The frozen biopsy duration varies from person to person and may vary from 20 minutes to 1 hour at the institution that conducted the study. Therefore, it was considered that the difference in the time taken for surgery between the control and Mediclore groups was attributed to the time spent during frozen biopsy, not the time taken while applying Mediclore.
The amount of tissue fluid drainage after surgery was significantly higher for the Mediclore group (89.1 ± 42.1 mL) than for the control group (60.8 ± 25.6 mL). Previous studies analyzing the efficacy of hyaluronic acid-carboxymethylcellulose solution in thyroid surgery have reported that the increase in total drainage after thyroid surgery depends on sex, weight, and body mass index, not on the use of the solution [23]. Despite the difference in the amount of drainage between the 2 groups, the postoperative discharge rate was the same for both groups, and there was no postoperative seroma in this study. Considering these results, the use of Mediclore is not expected to have a significant effect on patient progress. A systematic study is needed in the future.
This study had some limitations. First, there was no objective standard for evaluating the degree of adhesion; therefore, this study used a questionnaire with a 5-step evaluation scale to evaluate the degree of adhesion between patients and physicians. Although some studies have attempted to evaluate the degree of adhesion by objectively evaluating the movement of the esophagus, it has not yet been recognized as a standard evaluation index [618]. As there is no standard for objective indicators for evaluating adhesions after thyroid surgery, several studies have used surveys to determine the degree of adhesion [8131420]. Therefore, it is necessary to proceed according to the objective adhesion-evaluation criteria after thyroid surgery in the future.
The best way to evaluate postoperative adhesion is to evaluate the degree of adhesion during reoperation. Although this study was designed to evaluate adhesions in patients with reoperation, the number of patients who underwent completion surgery was very small. As differentiated thyroid cancer has a good prognosis, the 2015 American Thyroid Association guidelines recommend lobectomy rather than total thyroidectomy for less aggressive thyroid cancer [24]. Therefore, a large-scale study with a systematic and sufficient follow-up period is needed for more detailed analyses of the effect of anti-adhesion agent use on reoperation.
To conclude, Mediclore can be safely used as an anti-adhesive barrier. However, this study did not reveal its efficacy for postoperative adhesions. A more objective and systematic study is required in the future.
ACKNOWLEDGEMENTS
We would like to thank the patients who were willing to participate in the study and the Seoul National University Hospital.
Notes
References
1. Jung KW, Won YJ, Hong S, Kong HJ, Lee ES. Prediction of cancer incidence and mortality in Korea, 2020. Cancer Res Treat. 2020; 52:351–358. PMID: 32178488.

2. Thompson LD, Wieneke JA, Paal E, Frommelt RA, Adair CF, Heffess CS. A clinicopathologic study of minimally invasive follicular carcinoma of the thyroid gland with a review of the English literature. Cancer. 2001; 91:505–524. PMID: 11169933.

3. Mazzaferri EL, Young RL. Papillary thyroid carcinoma: a 10 year follow-up report of the impact of therapy in 576 patients. Am J Med. 1981; 70:511–518. PMID: 7211893.

4. Berber E, Bernet V, Fahey TJ 3rd, Kebebew E, Shaha A, Stack BC Jr, et al. American Thyroid Association statement on remote-access thyroid surgery. Thyroid. 2016; 26:331–337. PMID: 26858014.

5. Pan JH, Zhou H, Zhao XX, Ding H, Wei L, Qin L, et al. Robotic thyroidectomy versus conventional open thyroidectomy for thyroid cancer: a systematic review and meta-analysis. Surg Endosc. 2017; 31:3985–4001. PMID: 28337546.

6. Kim WY, Lee JB, Kim HY, Park PJ, Jung SP, Lee HY, et al. Prospective, randomized, double blind, multicenter study for an auto crosslinked polysaccharide gel to evaluate antiadhesive effect and safety compared to poloxamer/sodium alginate after thyroidectomy. Int Surg. 2019; 103:452–460.

7. Oh A. Trends of anti-adhesion adjuvant-review. Biomater Res. 2013; 17:138–145.
8. Park WS, Chung YS, Lee KE, Kim HY, Choe JH, Koh SH, et al. Anti-adhesive effect and safety of sodium hyaluronate and sodium carboxymethyl cellulose solution in thyroid surgery. Asian J Surg. 2010; 33:25–30. PMID: 20497879.

9. Kim YI, Lee M, Kim SI, Seol A, Lee EJ, Kim HS, et al. A randomized controlled trial of thermo-sensitive sol-gel anti-adhesion agent after gynecologic surgery. J Clin Med. 2020; 9:2261.

10. Kim TI, Jung W, Chung JY, Jeong H, Kim SH. Effect of a poloxamer-based thermosensitive gel on rotator cuff repair in a rabbit model: a controlled laboratory study. J Orthop Surg Res. 2019; 14:190. PMID: 31238965.

11. Chung JH, Kim KS, Choi JD, Kim TH, Lee KS, Oh CY, et al. Effects of poloxamer-based thermo-sensitive sol-gel agent on urethral stricture after transurethral resection of the prostate for benign prostatic hyperplasia: a multicentre, single-blinded, randomised controlled trial. BJU Int. 2020; 125:160–167. PMID: 31444917.

12. Nam IC, Joo YH, Cho JH, Kim CS, Kim SY, Kim GJ, et al. Effects of an antiadhesive agent on functional recovery of the greater auricular nerve after parotidectomy: a double-blind randomized controlled trial. Eur Arch Otorhinolaryngol. 2019; 276:3185–3193. PMID: 31338575.

13. Bae DS, Woo JW, Paek SH, Kwon H, Chai YJ, Kim SJ, et al. Antiadhesive effect and safety of sodium hyaluronate-carboxymethyl cellulose membrane in thyroid surgery. J Korean Surg Soc. 2013; 85:199–204. PMID: 24266009.

14. Park KS, Lee KE, Ku do H, Kim SJ, Park WS, Kim HY, et al. Antiadhesive effect and safety of oxidized regenerated cellulose after thyroidectomy: a prospective, randomized controlled study. J Korean Surg Soc. 2013; 84:321–329. PMID: 23741689.

15. Fischer A, Koopmans T, Ramesh P, Christ S, Strunz M, Wannemacher J, et al. Post-surgical adhesions are triggered by calcium-dependent membrane bridges between mesothelial surfaces. Nat Commun. 2020; 11:3068. PMID: 32555155.

16. Choi GJ, Park HK, Kim DS, Lee D, Kang H. Effect of statins on experimental postoperative adhesion: a systematic review and meta-analysis. Sci Rep. 2018; 8:14754. PMID: 30283040.

17. Lee JS, Kim JP, Ryu JS, Woo SH. Effect of wound massage on neck discomfort and voice changes after thyroidectomy. Surgery. 2018; 164:965–971. PMID: 30054014.

18. Alkan Z, Yigit O, Adatepe T, Uzun N, Kocak I, Sunter V, et al. Effect of anti-adhesive barrier use on laryngotracheal movement after total thyroidectomy: an electrophysiological study. Indian J Otolaryngol Head Neck Surg. 2014; 66(Suppl 1):71–77. PMID: 24533362.

19. Yigit O, Uslu Coskun B, Coskun H, Yilmaz B, Alkan S, Cinar U, et al. Efficacy of anti-adhesive barriers in secondary thyroidectomy: an experimental study. Laryngoscope. 2004; 114:1668–1673. PMID: 15475802.

20. Kim DY, Kang SW, Kim DS, Shin JU, Chung WY, Park CS, et al. Preventive effect of human acellular dermal matrix on post-thyroidectomy scars and adhesions: a randomized, double-blinded, controlled trial. Dermatol Surg. 2015; 41:812–820. PMID: 26066615.

21. Andres Y, Giraud L, Gerente C, Le Cloirec P. Antibacterial effects of chitosan powder: mechanisms of action. Environ Technol. 2007; 28:1357–1363. PMID: 18341146.

22. Raafat D, von Bargen K, Haas A, Sahl HG. Insights into the mode of action of chitosan as an antibacterial compound. Appl Environ Microbiol. 2008; 74:3764–3773. PMID: 18456858.

23. Kim HK, Kim SM, Chang H, Chun KW, Kim BW, Lee YS, et al. Anti-adhesive agent (Guardix-SG®) does not influence the drainage volume after thyroid cancer surgery. Korean J Endocr Surg. 2013; 13:251.

24. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016; 26:1–133. PMID: 26462967.

SUPPLEMENTARY MATERIALS
Supplementary Tables 1 and 2 can be found via https://doi.org/10.4174/astr.2022.102.6.313.
Supplementary Table 1
The difference in operating time according to frozen biopsy between the control and Mediclore
groups
Supplementary Table 2
The outcomes of adhesion-evaluation score during completion thyroidectomy
Fig. 1
Flow diagram of the randomized controlled trial. The thermosensitive sol-gel agent was applied to the surgical site in the Mediclore group. No anti-adhesive agent was applied to the surgical site. Mediclore: CGBio Co., Ltd. Seongnam, Korea.
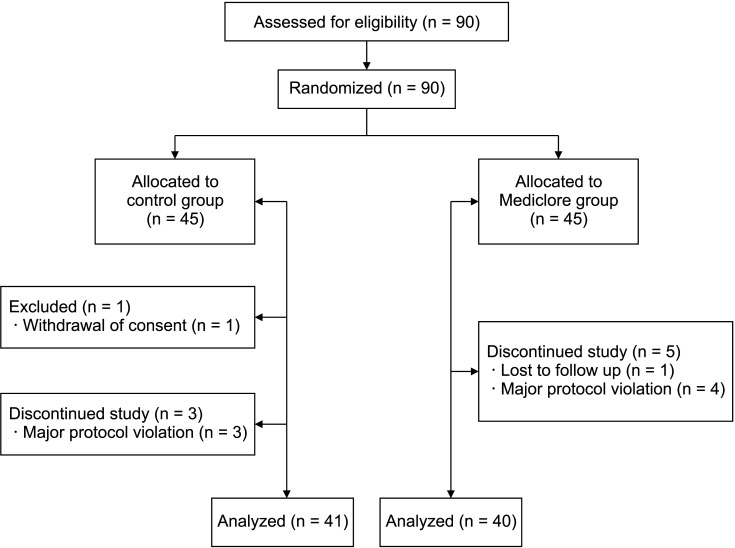
Fig. 2
Comparison of swallowing difficulty by group and time. There was no difference in the degree of difficulty swallowing between the 2 groups based on the questionnaire survey of the postoperative adhesions at 2 weeks, 3 months, and 6 months after surgery. Mediclore: CGBio Co., Ltd. Seongnam, Korea.
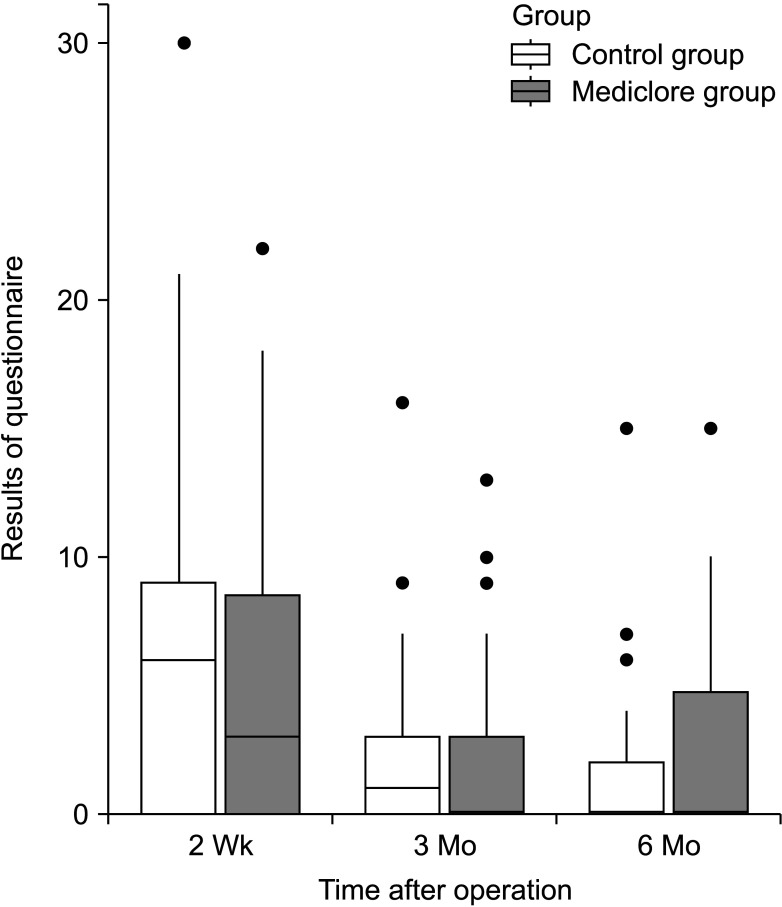
Fig. 3
Comparison of inflammatory problems by group and time. There was no difference in the outcomes between the 2 groups when the wrinkle problems were assessed using the questionnaire for postoperative adhesions 2 weeks, 3 months, and 6 months after surgery. Mediclore: CGBio Co., Ltd. Seongnam, Korea.
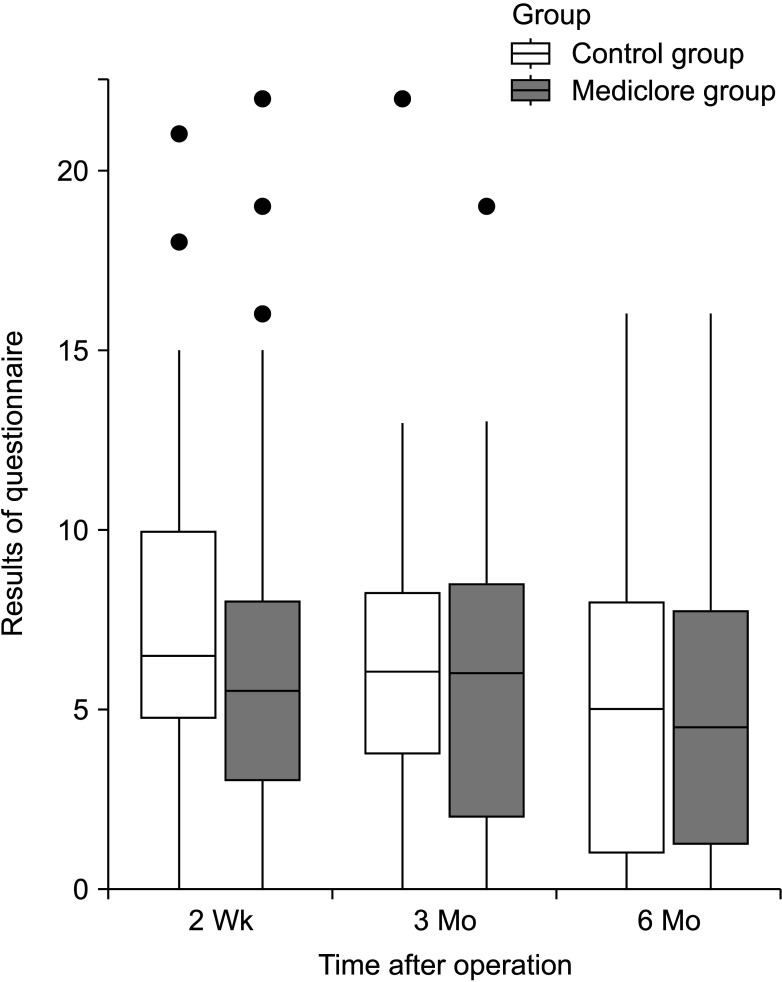
Fig. 4
Comparison of wrinkle problems by group and time. There was no difference in the scores for inflammatory problems at 2 weeks, 3 months, and 6 months after surgery. Mediclore: CGBio Co., Ltd. Seongnam, Korea.
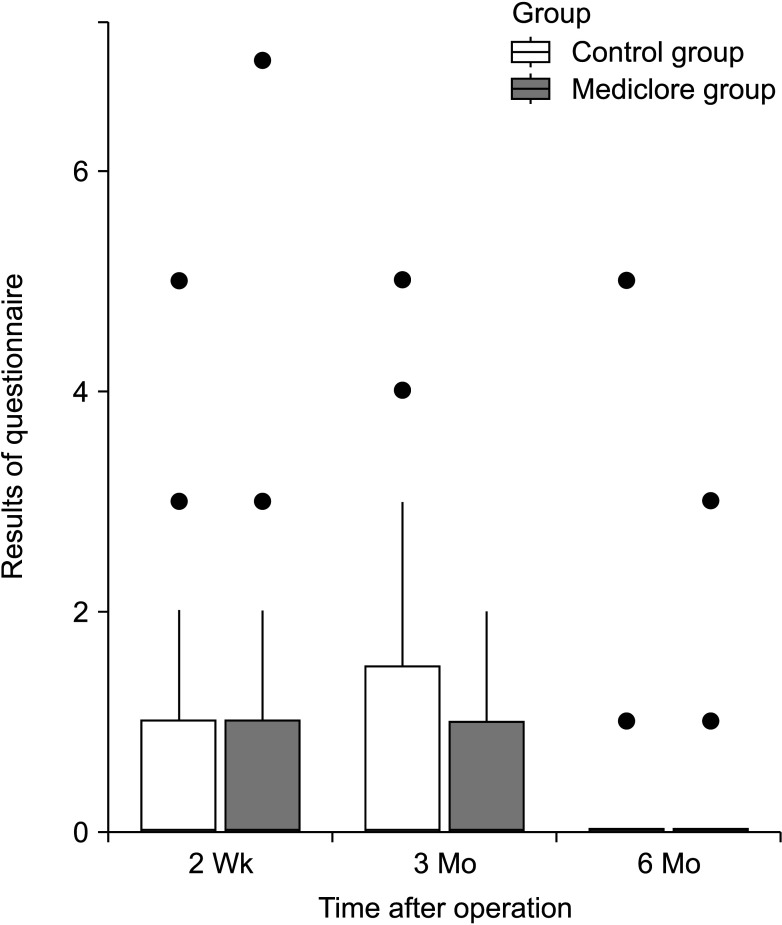
Table 2
Demographic and clinical characteristics of the study cohort

Values are presented mean ± standard deviation or number (%).
CND, central compartment lymph node dissection; RLN, recurrent laryngeal nerve.
Mediclore: CGBio Co., Ltd. Seongnam, Korea.
a)Drain refers to the total amount of postoperative drainage during hospitalization. b)Postoperative complications were divided into transient and permanent groups based on the duration of complications at 6 months. c)Postoperative complication was analyzed statistically, excluding diseases that did not occur.




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download