Abstract
Purpose
Microinvasive breast cancer (MIBC) is an invasive carcinoma with a tumor dimension not exceeding 1 mm. Owing to its low incidence, the rate of axillary node metastasis and its management are not well established. The aim of this study was to assess the incidence of lymph node metastasis (LNM) and identify variables associated with LNM, as well as to evaluate the need for axillary staging in MIBC patients by analyzing nationwide data.
Methods
The Korean Breast Cancer Society registry was searched to identify MIBC patients diagnosed between January 1996 and April 2020. Patients without neoadjuvant chemotherapy experiences, systemic metastasis, and missing or discordant data were eligible for the analysis. The incidence rate of LNM was determined, and variables associated with LNM were identified by multivariable regression analysis.
Results
Of 2,427 MIBC patients identified, 98 (4.0%) had LNM and 12 (0.5%) had N2/3 disease. Type of breast operation (odds ratio [OR], 2.093; 95% confidence interval [CI], 1.332–3.290; P = 0.001), age (OR, 2.091; 95% CI, 1.326–3.298; P = 0.002), hormone receptor status (OR, 2.220; 95% CI, 1.372–3.594; P = 0.001), and lymphovascular invasion (OR, 11.143; 95% CI, 6.354–19.540; P < 0.001) were significantly related to LNM.
Conclusion
The incidence of LNM in MIBC patients was only 4.0% in our study, suggesting that de-escalation of axillary surgical interventions could be carefully considered. The indications for axillary staging should be individualized considering tumor volume, age, hormone receptor status, and lymphovascular invasion to improve the quality of life of MIBC survivors.
Microinvasive breast cancer (MIBC) is an invasive carcinoma in which the greatest tumor dimension does not exceed 1 mm (T1mi) and it accounts for 0.68%–2.4% of all breast cancers worldwide [12]. In 1996, the Union for International Cancer Control first included T1mi in the TNM classification system of breast cancer, and MIBC became characterized as a separate entity [3]. MIBC is not a common subgroup of breast cancer, and it represents a pathological transitional state between ductal carcinoma in situ (DCIS) and invasive breast cancer [4].
Because of the low incidence of MIBC, the associated incidence of axillary node metastasis and management strategies for microinvasive carcinoma are not well established, although they are most likely between those for DCIS and invasive breast carcinomas [56]. MIBC is usually diagnosed after the surgical treatment of DCIS patients. Kotani et al. [7] reported that 20% of patients with a suspicion of DCIS on biopsy were upstaged to invasive breast cancer postoperatively. The rate of lymph node (LN) metastasis (1%) was comparable with the rate of nodal metastasis of DCIS, and there is insufficient evidence to support performing sentinel LN biopsy (SLNB) at the time of initial surgery [8]. However, patients postoperatively diagnosed with MIBC require an additional operation for LN staging [9].
Axillary LN staging is associated with sequelae such as lymphedema and chronic pain, as well as stiffness of the arm and shoulder, which interfere with daily living activities and quality of life [1011]. Considering the low rate of LN metastasis and possible complications of axillary staging, the de-escalation of axillary LN staging may be considered when designing treatment plans for MIBC patients [12]. The primary aim of this study was to assess the incidence of LN metastasis and to evaluate the need for axillary staging in MIBC patients by analyzing nationwide data. The secondary aim was to identify variables associated with LN metastasis in MIBC patients.
This study was approved by the Institutional Review Board of the Seoul St. Mary’s Hospital (No. KC20ZASI1001), and the Board waived the requirement for informed consent.
The data of 190,049 breast cancer patients from the online Korean Breast Cancer Society (KBCS) registry database were retrospectively reviewed. Nationwide breast cancer data have been analyzed and reported by the KBCS since 1996 for the evaluation of chronological changes in Korean breast cancer patients [13]. Thirty-eight of the 41 medical schools in Korea and more than 100 hospitals, including 74 university hospitals, 24 general hospitals, and 6 private breast clinics, voluntarily participated in the program, and the database was established online [14]. The database provides clinicopathological factors, such as age; sex; type of surgery; breast cancer stage (according to the 8th American Joint Committee on Cancer classification); family history; parity; age of menarche; histology; presence of the biological markers estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2); methods of treatment; types of chemotherapy; and date and cause of death [14].
A total of 190,049 breast cancer patients who were surgically treated between January 1978 and April 2020 in Korea were identified from the KBCS registry database. Patients diagnosed before 1997 were excluded because T1mi was clearly defined in 1996 [3]. Patients who were treated with neoadjuvant chemotherapy, those with de novo stage IV disease, and patients with missing or discordant data were also excluded from the study. A total of 2,427 MIBC patients were ultimately eligible for the analysis (Fig. 1).
The collected clinical and histopathologic variables included age, sex, body mass index, family history of breast cancer, symptoms before surgery (palpable mass, pain, nipple retraction, skin changes, nipple discharge, and axillary mass), pathology results (histologic type and grade, nuclear grade), hormone receptor status, HER2 status, Ki-67 results, lymphovascular invasion (LVI), extensive intraductal component, type of surgery, and axillary LN staging.
The chi-square test or Fisher exact test was used to evaluate the relationships between groups.
The associations between the clinical and histopathological characteristics and node positivity were analyzed using binominal logistic regression model univariate and multivariate analyses. The variables that were statistically significantly associated (P < 0.05) with LN metastasis in the univariate analysis were included in the multivariate analysis. The hazard ratios (HRs), 95% confidence intervals (CIs), and the P-values were calculated. The statistical analyses were performed using IBM SPSS Statistics ver. 24 (IBM Corp., Armonk, NY, USA).
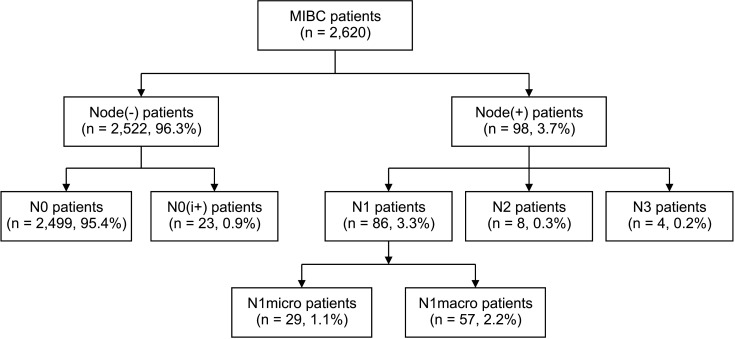
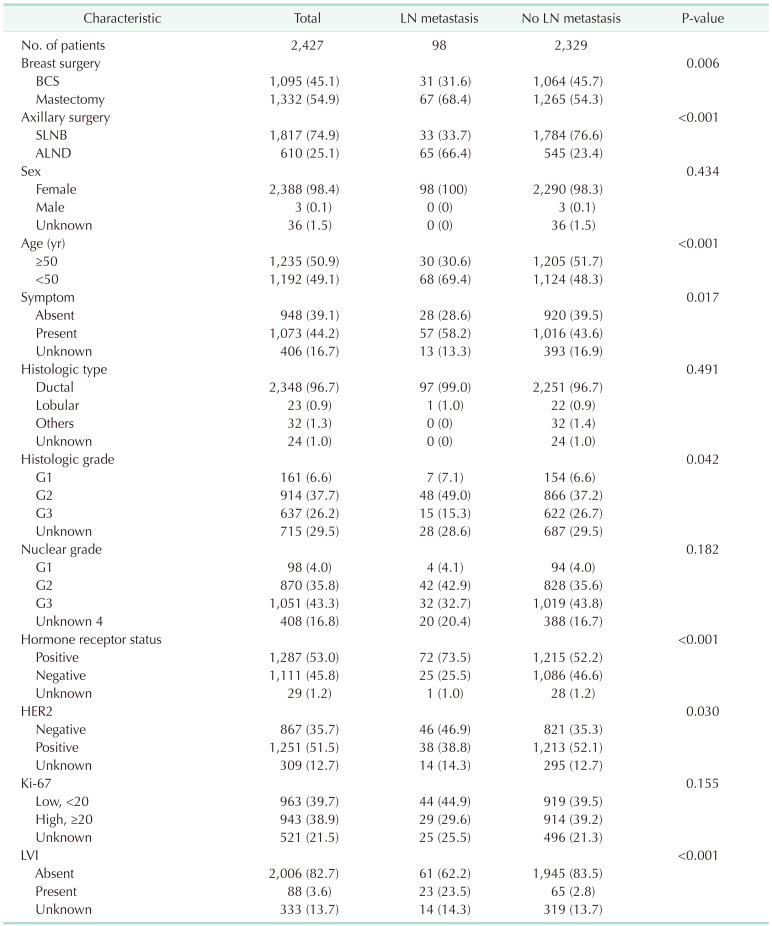
Of 2,427 MIBC patients included in the study, 98 (4.0%) had LN metastasis, including both macro- and micrometastasis (Fig. 2). Of the 98 patients with LN metastasis, 8 (0.3%) were pN2 and 4 (0.2%) were pN3. The median age of the patients with LN metastasis was 45 years (range, 28–76 years), and that of the patients without LN metastasis was 50 years (range, 22–89 years; P < 0.001) (Table 1). Patients with LN metastasis were more likely to undergo total mastectomy (68.4% vs. 54.3%, P = 0.006) and axillary LN dissection (66.4% vs. 23.4%, P < 0.001) and have symptoms related to breast cancer than those without LN metastasis (58.2% vs. 43.6%, P = 0.017). Patients with LN metastasis were more likely to have hormone receptor-positive tumors (73.5% vs. 52.2%, P < 0.001) and show LVI (23.5% vs. 2.8%, P < 0.001), but less likely to have HER2 overexpressed tumors (38.8% vs. 52.1%, P = 0.030) than those without LN metastasis. There were no significant differences in family history of breast cancer, histologic type, or Ki-67 level between patients with LN metastasis and those without.
The clinicopathologic factors related to LN metastasis were reproduced in the univariate logistic regression analysis (Table 2). Multivariate analysis showed that type of breast operation (odds ratio [OR], 2.093; 95% CI, 1.332–3.290; P = 0.001), age (OR, 2.091; 95% CI, 1.326–3.298; P = 0.002), hormone receptor status (ER and PR positive; OR, 2.220; 95% CI, 1.372–3.594; P = 0.001) and LVI (OR, 11.143; 95% CI, 6.354–19.540; P < 0.001) are independent predictors of LN metastasis and LVI was the most powerful variant affecting it.
This study was a large scale, population-based, nationwide study that investigated the incidence and risk factors of LN metastasis in MIBC. Among 2,427 MIBC patients, 4% of patients presented with LN metastasis in this study. Patients younger with positive HR and LVI who received mastectomies were more likely to present with LN metastasis. The low incidence rate of LN metastasis in this study is consistent with the results of previous nationwide studies. A Danish nationwide study analyzed 233 patients, of whom 27 (11.6%) had LN metastasis including micrometastasis [9]. A study using the Surveillance, Epidemiology, and End Results database showed that 7.6% of patients had LN metastasis (n = 8,863) [15], whereas data from the National Cancer Database reported a rate of LN metastasis of 2.9% (n = 2,609) [16].
Clinicopathological characteristics, such as younger age, positive ER status, positive HER2 status, large volume of in situ disease (>5 cm), histologic type of breast cancer, higher histologic grade, black ethnicity, larger tumor size, presence of necrosis, and LVI, have been suggested as risk factors associated with LN metastasis in MIBC patients [6912171819]. Among these characteristics, we were able to confirm younger age, positive hormone receptor status, and LVI as significant risk factors associated with LN metastases.
The association of LN metastasis with younger age and LVI is consistent with previous literature [691718]. In a Danish nationwide study, age of <50 years was one of the 2 risk factors associated with LN metastasis, and an Italian multicenter study reported that age of <60 years was a risk factor for LN metastasis [918]. A study by Gooch et al. [6] showed that LVI was independently related to LN metastasis and was the strongest factor affecting the incidence of LN metastasis in MIBC patients, which is consistent with the present results.
The relationship between hormone receptor status and LN metastasis has not been widely investigated, and the results are under debate. Kapoor et al. [19] demonstrated that ER-negative cancers are significantly associated with LN metastasis, whereas a retrospective study by Ko et al. [17] suggested that positive ER status was independently related to LN metastasis. In this study, hormone receptor-positive patients had an increased risk of LN metastasis. Investigation of the biological behavior of MIBC is limited because the volume of the invasive component of MIBC is small, which could lead to inconsistencies in the results.
Although the size of DCIS was not provided in the KBCS registry database, patients undergoing mastectomy can be assumed to have a larger tumor size compared to patients undergoing breast conserving surgery. The higher rate of LN metastasis in MIBC patients undergoing mastectomy can be explained by its larger tumor size, as previous literatures identified large tumor size as a risk factor for LN metastasis in MIBC patients [612].
The low rate of LN metastasis in MIBC demonstrated in this study suggests that a de-escalation of axillary LN staging should be considered. Previous studies suggested that LN staging should be avoided or individualized for MIBC patients [89171820]. The diagnosis of MIBC is usually made after surgical treatment in DCIS patients, and a secondary operation is frequently required for LN staging. Although SLNB is a safe procedure, it is associated with complications such as pain, mobility restriction, and lymphedema, which are long-term risks [20]. In view of the low rate of LN metastasis in MIBC and the complications of axillary staging, SLNB omission could be considered in MIBC patients. However, an individualized approach is needed, as suggested by the high axillary LN burden identified in this cohort. Clinical trials, such as NAUTILUS trial (No Axillary Surgical Treatment in Clinically Lymph Node-Negative Patients after Ultrasonography trial; NCT04303715, https://clinicaltrials.gov/ct2/show/NCT04303715) and the SOUND trial (Sentinel node vs. Observation after axillary UltrasouND trial; NCT02167490, https://clinicaltrials.gov/ct2/show/NCT02167490), are ongoing to investigate the non-inferiority of SLNB omission in clinically node-negative patients. These studies will clarify whether routine SLNB omission is possible and under which circumstances. Until then, the indications for axillary staging in MIBC patients could be individualized considering age, hormone receptor status, and LVI status for each patient.
The present study had several limitations. The study is a retrospective analysis, which may lead to a fundamental bias, although the data from the KBCS registry is prospectively maintained. In addition, certain variables related to LN metastasis, such as DCIS size or axillary LN status on imaging studies, were not included. Additional studies including imaging studies are needed, in particular for considering SLNB omission.
This study was the largest study confirming the low incidence of LN metastasis (3.7%) in MIBC patients in the Asian population and identifying variables responsible for LN positivity. The low incidence of LN-positive patients with MIBC demonstrated in this study suggests that de-escalation of axillary surgical interventions could be carefully considered in MIBC patients to improve the quality of life of MIBC survivors and to reduce the morbidity from axillary operations.
ACKNOWLEDGEMENTS
We wish to thank Dr. HeeWon Seo and Dr. Hocheol Lee for statistical analyses. This article was supported by the Korean Breast Cancer Society. We thank all members of the KBCS who participated in the KBCS online registry.
References
1. Giuliano AE, Connolly JL, Edge SB, Mittendorf EA, Rugo HS, Solin LJ, et al. Breast Cancer-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017; 67:290–303. PMID: 28294295.

2. Hoda SA, Chiu A, Prasad ML, Giri D, Hoda RS. Are microinvasion and micrometastasis in breast cancer mountains or molehills? Am J Surg. 2000; 180:305–308. PMID: 11113441.

3. Hermanek P, Hutter RVP, Sobin LH, Wagner G, Wittekind CH. TNM Atlas: illustrated guide to the TNM/p TNM classification of malignant tumors. 4th ed. Berlin: Springer-Verlag;1997.
4. Kuerer HM, Smith BD, Chavez-MacGregor M, Albarracin C, Barcenas CH, Santiago L, et al. DCIS margins and breast conservation: MD Anderson Cancer Center multidisciplinary practice guidelines and outcomes. J Cancer. 2017; 8:2653–2662. PMID: 28928852.

5. Magnoni F, Massari G, Santomauro G, Bagnardi V, Pagan E, Peruzzotti G, et al. Sentinel lymph node biopsy in microinvasive ductal carcinoma in situ. Br J Surg. 2019; 106:375–383. PMID: 30791092.
6. Gooch JC, Schnabel F, Chun J, Pirraglia E, Troxel AB, Guth A, et al. A nomogram to predict factors associated with lymph node metastasis in ductal carcinoma in situ with microinvasion. Ann Surg Oncol. 2019; 26:4302–4309. PMID: 31529311.

7. Kotani H, Yoshimura A, Adachi Y, Ishiguro J, Hisada T, Ichikawa M, et al. Sentinel lymph node biopsy is not necessary in patients diagnosed with ductal carcinoma in situ of the breast by stereotactic vacuum-assisted biopsy. Breast Cancer. 2016; 23:190–194. PMID: 24989112.

8. Flanagan MR, Stempel M, Brogi E, Morrow M, Cody HS 3rd. Is sentinel lymph node biopsy required for a core biopsy diagnosis of ductal carcinoma in situ with microinvasion? Ann Surg Oncol. 2019; 26:2738–2746. PMID: 31147995.

9. Holm-Rasmussen EV, Jensen MB, Balslev E, Kroman N, Tvedskov TF. Sentinel and non-sentinel lymph node metastases in patients with microinvasive breast cancer: a nationwide study. Breast Cancer Res Treat. 2019; 175:713–719. PMID: 30877405.

10. Gutman H, Kersz T, Barzilai T, Haddad M, Reiss R. Achievements of physical therapy in patients after modified radical mastectomy compared with quadrantectomy, axillary dissection, and radiation for carcinoma of the breast. Arch Surg. 1990; 125:389–391. PMID: 2306186.

11. Verbelen H, Gebruers N, Eeckhout FM, Verlinden K, Tjalma W. Shoulder and arm morbidity in sentinel node-negative breast cancer patients: a systematic review. Breast Cancer Res Treat. 2014; 144:21–31. PMID: 24496928.

12. Lillemoe TJ, Tsai ML, Swenson KK, Susnik B, Krueger J, Harris K, et al. Clinicopathologic analysis of a large series of microinvasive breast cancers. Breast J. 2018; 24:574–579. PMID: 29476574.

13. Min SY, Kim Z, Hur MH, Yoon CS, Park EH, Jung KW, et al. The basic facts of Korean breast cancer in 2013: results of a nationwide survey and Breast Cancer Registry database. J Breast Cancer. 2016; 19:1–7. PMID: 27066090.

14. Park S, Moon BI, Oh SJ, Lee HB, Seong MK, Lee S, et al. Clinical subtypes and prognosis in breast cancer according to parity: a nationwide study in Korean Breast Cancer Society. Breast Cancer Res Treat. 2019; 173:679–691. PMID: 30390214.

15. Wang W, Zhu W, Du F, Luo Y, Xu B. The demographic features, clinicopathological characteristics and cancer-specific outcomes for patients with microinvasive breast cancer: a SEER database analysis. Sci Rep. 2017; 7:42045. PMID: 28165014.

16. Fan B, Pardo JA, Serres S, Alapati AC, Szewczyk J, Mele A, et al. Role of sentinel lymph node biopsy in microinvasive breast cancer. Ann Surg Oncol. 2020; 27:4468–4473. PMID: 32430750.

17. Ko BS, Lim WS, Kim HJ, Yu JH, Lee JW, Kwan SB, et al. Risk factor for axillary lymph node metastases in microinvasive breast cancer. Ann Surg Oncol. 2012; 19:212–216. PMID: 21633867.

18. Fortunato L, Santoni M, Drago S, Gucciardo G, Farina M, Cesarini C, et al. Sentinel lymph node biopsy in women with pT1a or “microinvasive” breast cancer. Breast. 2008; 17:395–400. PMID: 18468896.

19. Kapoor NS, Shamonki J, Sim MS, Chung CT, Giuliano AE. Impact of multifocality and lymph node metastasis on the prognosis and management of microinvasive breast cancer. Ann Surg Oncol. 2013; 20:2576–2581. PMID: 23468047.

20. Phantana-Angkool A, Voci AE, Warren YE, Livasy CA, Beasley LM, Robinson MM, et al. Ductal carcinoma in situ with microinvasion on core biopsy: evaluating tumor upstaging rate, lymph node metastasis rate, and associated predictive variables. Ann Surg Oncol. 2019; 26:3874–3882. PMID: 31342378.

Fig. 1
Flowchart of the microinvasive breast cancer (MIBC) patients included in our study from the Korean Breast Cancer Society (KBCS) registry database KBCS.
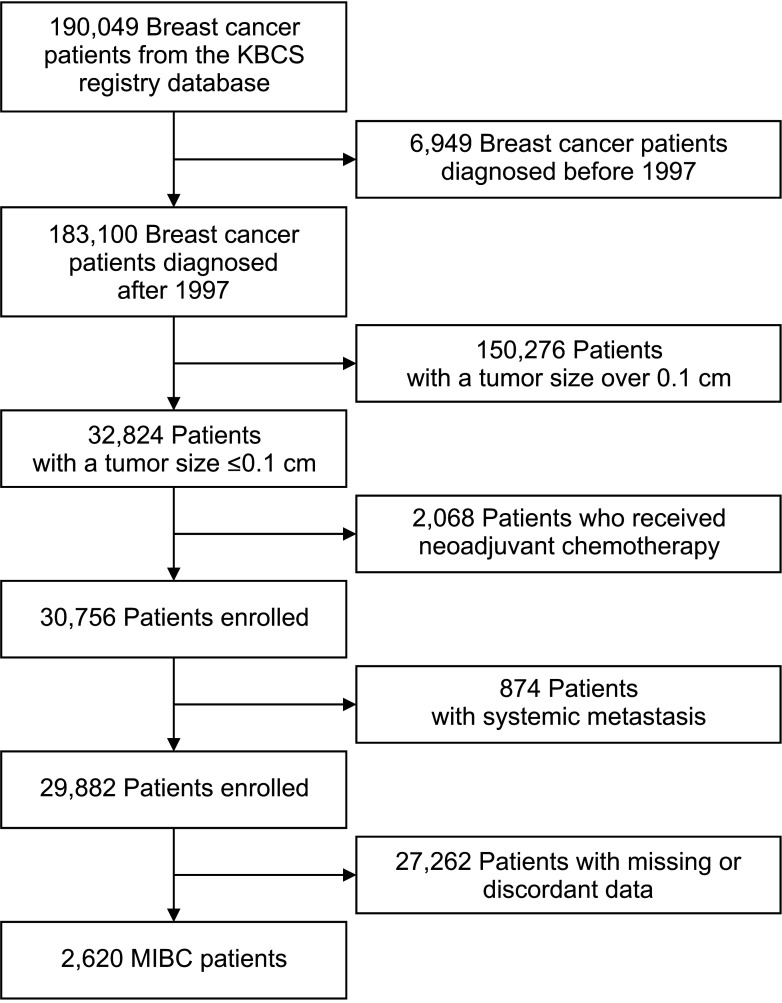
Fig. 2
Flowchart of lymph node metastasis in microinvasive breast cancer (MIBC) patients treated between 1997 and 2020. N0(i+), N isolated tumor cell clusters; N1micro, N1 micrometastasis; N1macro, N1 macrometastasis.




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download