Abstract
Purpose
Malignant intraductal papillary neoplasm of the bile duct of the liver (IPNB-L) cannot readily be diagnosed through preoperative CT or MRI, but fluorodeoxyglucose (FDG)-PET is a viable alternative. This study evaluated the diagnostic and prognostic impacts of FDG-PET in patients with IPNB-L.
Methods
This was a retrospective single-center study of 101 IPNB-L patients who underwent hepatectomy between 2010 and 2019.
Results
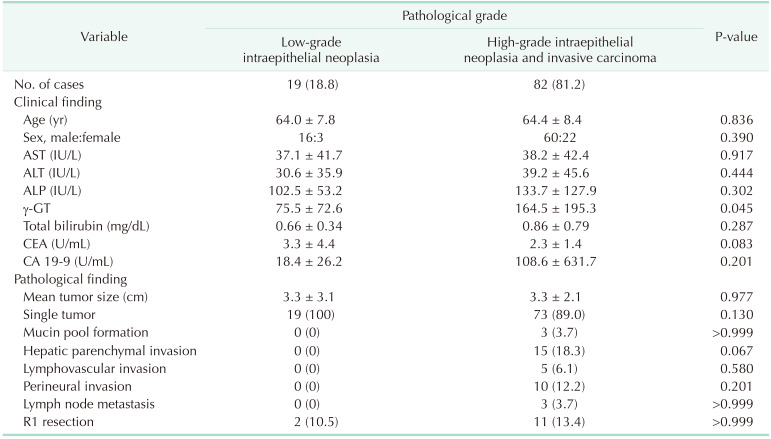
Mean age was 64.4 ± 8.3 years and 76 (75.2%) were male. Anatomical hepatic resection was performed in 99 (98.0%). Concurrent bile duct resection and pancreaticoduodenectomy were performed in 41 (40.6%) and 1 (1.0%), respectively. R0 and R1 resections were performed in 88 (87.1%) and 13 (12.9%), respectively. Low-grade intraepithelial neoplasia and high-grade neoplasia/invasive carcinoma were diagnosed in 19 (18.8%) and 82 (81.2%), respectively. Median FDG-PET maximal standardized uptake values (SUVmax) in low-grade neoplasia and high-grade neoplasia/carcinoma were 3.6 (range, 1.7–7.6) and 5.2 (range, 1.5–18.7) (P = 0.019), respectively. Receiver operating characteristic curve analysis of SUVmax showed area under the curve of 0.674, with sensitivity of 84.2% and specificity of 47.4% at SUVmax cutoff of 3.0. This cutoff had no significant influence on tumor recurrence (P = 0.832) or patient survival (P = 0.996) in patients with IPNB-L of high-grade neoplasia or invasive carcinoma.
Conclusion
IPNB-L is a rare type of biliary neoplasm and encompasses a histological spectrum ranging from benign disease to invasive carcinoma. An FDG-PET SUVmax cutoff of 3.0 appears to effectively discern high-grade neoplasia/carcinoma from low-grade neoplasia, which will assist with the surgical strategy for these cases.
Intraductal papillary neoplasm of the bile duct (IPNB) is a rare disease with a reported prevalence of 4%–38% among all bile duct tumors [1234]. This disease was classified as a distinct clinical and pathological entity by the 2010 and 2019 World Health Organization classification systems [56]. IPNB is known as one of the premalignant lesions of cholangiocarcinoma and has a wide histological range including low-grade and high-grade intraepithelial neoplasia (HGIEN), and IPNB with invasive carcinoma. The pathological features of IPNBs are heterogeneous and include various types of intra- and extrahepatic intraductal bile duct tumors. The postresection prognosis of IPNB depends on its histopathological grades, and an accurate preoperative diagnosis of IPNB with HGIEN and invasive carcinoma is thus needed to design the optimal surgical strategy. Although CT and MRI are frequently used to diagnose IPNB, the preoperative identification of IPNB with HGIEN and invasive carcinoma using these modalities remains challenging [7]. Fluorodeoxyglucose (FDG)-PET is reported to be useful for identifying IPNB with invasive carcinoma [8]. The preoperative prediction of IPNB with HGIEN and invasive carcinoma is usually difficult with CT and MRI, particularly for IPNB of the liver (IPNB-L). The aim of this study was to evaluate the diagnostic and prognostic impact of FDG-PET in patients with IPNB-L.
The study protocol was approved by the Institutional Review Board at of Asan Medical Center (No. 2021-1347), which waived the requirement for informed consent due to the retrospective nature of this study. This study was performed in accordance with the ethical guidelines of the World Medical Association Declaration of Helsinki 2013.
This was a retrospective single-center study. The primary purpose of the present study was to evaluate the diagnostic impact of FDG-PET for discrimination of malignant from benign IPNB-L. The secondary purpose was to assess the prognostic impact of FDG-PET on the long-term postoperative outcomes of patients with IPNB-L of HGIEN and invasive carcinoma.
We searched for patients who were diagnosed with IPNB-L in our institutional liver resection database during a 10-year study period between January 2010 and December 2019. This study was focused on IPNB-L, thus patients with only extrahepatic IPNB were excluded. We only included IPNB-L cases who had undergone FDG-PET within 1 month prior to their operation from which standardized uptake values (SUV) had been recorded. Finally, a total of 101 patients were selected as the study group. They were followed up until July 2021 or patient death through a review of our institutional medical records and with the assistance of the National Health Insurance Service in Korea.
Routine preoperative imaging evaluations for the study patients included abdominal and chest CT, liver MRI with cholangiopancreatography (MRCP), and FDG-PET. Diagnostic modalities for bile duct evaluation included endoscopic retrograde cholangiopancreatography, MRCP, and percutaneous transhepatic cholangioscopy.
FDG-PET/CT scans were performed with a Biograph Sensation16 (BIO16) or TruePoint 40 (BIO40) system (Siemens Medical Systems, Knoxville, TN, USA), equipped with a 16-slice or 40-slice CT scanner, respectively. All patients fasted for 6 hours or longer and had a serum glucose concentration <150 mg/dL prior to FDG-PET scanning. Whole-body images were obtained 50–70 minutes after intravenous injection of 333–688 MBq (9.0–18.6 mCi) of FDG. CT scanning was performed in spiral mode from the skull base to the proximal thigh at 100 mA and 120 kV, with a section width of 5 mm and collimation of 0.75 mm. No oral or intravenous contrast medium was used for CT and the resulting data of which were processed for attenuation correction and image fusion. This was followed by 3-dimensional caudocranial PET emission scanning with an acquisition time of 2.5 minutes per bed position with 6 or 7 bed positions for the whole body, and 5 minutes per bed position with 2 bed positions for the head and neck.
The SUV was calculated for the quantitative analysis of the tumor FDG uptake as follows: SUV = C (kBq/mL)/injected dose (kBq)/body weight (kg), where C is the tissue activity concentration measured by PET. The maximal SUV (SUVmax) for the focal area of uptake, defined as the highest SUV in the pixel within the regions of interest, was measured.
Patients were followed every 2–4 months during the first year after surgery, depending on the pathology and tumor stage; and the follow-up interval was adjusted on a case-by-case basis thereafter. For patients with IPNB-L of HGIEN or invasive carcinoma, the follow-up interval was set at 3–4 months up to 5 years and thereafter interval was prolonged until 10 years. The general principles of treatment for intrahepatic cholangiocarcinoma were adopted for all IPNB-L cases diagnosed with carcinoma [9].
Categorical variables were compared using the chi-square test or Fisher exact test. Continuous variables were expressed as a mean and standard deviation or median value with ranges, and analyzed with the Student t-test, Mann-Whitney U-test, or analysis of variance, depending on the distribution of the values. Survival curves were estimated by the Kaplan-Meier method and compared using the log-rank test. The SUVmax cutoff for predicting malignant IPNB-L was determined using receiver operating characteristic (ROC) curve analysis. The optimal SUVmax cutoff, sensitivity, and specificity were determined using the Youden index. An internal validation study was performed after dividing the malignant IPNB-L group into 2 subgroups according to the operation period (before and after the year 2014). A P-value of <0.05 was regarded as statistically significant. All statistical analyses were performed using IBM SPSS Statistics ver. 22 (IBM Corp., Armonk, NY, USA) and MedCalc ver. 20.010 (Ostend, Belgium).
Of the 101 patients with IPNB-L, there were 76 male patients (75.2%) and 25 female patients (24.8%). The mean patient age was 64.4 ± 8.3 years (range, 37–83 years). Eleven patients underwent prior cholecystectomy because of gallstone diseases. Intrahepatic duct stones were detected in 15 (14.9%) patients at the time of their IPNB-L diagnosis. The median concentration of CA 19-9 was 12.3 U/mL (range, 0.6–5,679.0 U/mL). The clinical profiles of the study patients are summarized in Table 1.
All patients underwent hepatic resection with or without concurrent bile duct resection. The extents of hepatectomy were right hepatectomy in 40, left hepatectomy in 46, right trisectionectomy in 2, central bisectionectomy in 2, right anterior sectionectomy in 3, right posterior sectionectomy in 4, left medial sectionectomy in 1, left lateral sectionectomy in 1, and segmentectomy and partial hepatectomy in 2. Concurrent bile duct resection and pancreaticoduodenectomy were performed in 41 (40.6%) and 1 patient (1.0%), respectively. R0 and R1 resections were performed in 88 (87.1%) and 13 patients (12.9%), respectively.
The histopathologic gradings of IPNB-L were low-grade intraepithelial neoplasia in 19 patients (18.8%), HGIEN in 32 patients (31.7%), and invasive carcinoma in 50 patients (49.5%). Thus, low-grade neoplasia and HGIEN/carcinoma lesions were diagnosed in 19 (18.8%) and 82 patients (81.2%), respectively. The mean tumor diameter was 3.3 ± 2.3 cm. Multiple tumors were found in 9 patients (8.9%). These pathological findings are summarized in Table 1.
The 1-, 3-, 5-, and 10-year tumor recurrence rates were 0%, 0%, 0%, and 25.0%, respectively, in the low-grade neoplasia IPNB-L group; and 11.1%, 18.3%, 29.9%, and 44.4%, respectively, in the HGIEN/carcinoma IPNB-L group (P = 0.019) (Fig. 1A).
The 1-, 3-, 5-, and 10-year tumor overall patient survival rates were all 100% in the low-grade neoplasia IPNB-L group; and 97.6%, 88.8%, 78.8%, and 48.5%, respectively, in the HGIEN/carcinoma IPNB-L group (P = 0.009) (Fig. 1B).
The distribution of the FDF-PET SUVmax values in accordance with the histopathological type of IPNB-L is presented in Fig. 2. The median values of the low-grade neoplasia and HGIEN/carcinoma IPNB-Ls were 3.6 (range, 1.7–7.6; 95% confidence interval [CI], 1.51–4.93) and 5.2 (range, 1.5–18.7; 95% CI, 4.10–6.27), respectively (P = 0.019).
ROC curve analysis of the FDG-PET SUVmax values calculated an area under the ROC curve of 0.674 (95% CI, 0.573–0.764; P = 0.015) (Fig. 3). The Youden index J was 0.315 (95% CI, 0.123–0.445) at an SUVmax cutoff of 3.0, which showed a sensitivity of 84.2% and a specificity of 47.4%. Precision-recall curve analysis indicated that the positive predictive values were well maintained at over 80% along with the changes in sensitivity (Fig. 4).
To assess the diagnostic reliability of an SUVmax cutoff value of 3.0, an internal validation study was performed after diving the HGIEN/carcinoma IPNB-L group into 2 subgroups according to the operation period (before and after the year 2014). The sensitivity and specificity of an SUVmax cutoff value of 3.0 for diagnosis of HGIEN/carcinoma IPNB-L were 73.2% and 47.4%, respectively, with 19 patients with low-grade neoplasia IPNB-L and early 41 patients with HGIEN/carcinoma IPNB-L; and 92.9% and 47.4%, respectively, with 19 patients with low-grade neoplasia IPNB-L and late 41 patients with HGIEN/carcinoma IPNB-L.
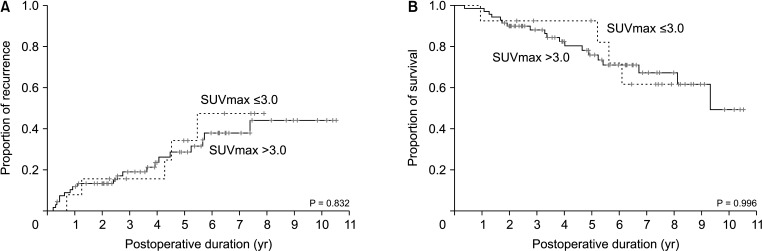
To assess the pathological difference according to SUVmax cutoff, the incidence of IPNB-L with carcinoma, high-grade neoplasia, and low-grade IPNB-Ls were 44, 25, and 10, respectively, for SUVmax of >3.0; and 6, 7, and 9, respectively, for SUVmax of ≤3.0 (P = 0.006).
IPNB cases have been reported sporadically around the world and the propensity of these lesions for malignant transformation has been well established [11011]. IPNB was proposed as a new disease entity in the 2010 World Health Organization (WHO) classification and remained in the 2019 WHO classification [56]. The incidence of IPNB is reported to range from 9.9% to 30% of bile duct tumors in Asian countries [1213], and from 7% to 11% of bile duct tumors in Western countries [1415]. Analysis of our institutional database revealed that IPNB-L accounted for approximately 13% of the intrahepatic bile duct tumor patients that had undergone hepatic resection.
An accurate preoperative diagnosis of IPNB-L is difficult in clinical practice because of the lack of specific symptoms and heterogenous extents of tumor growth. The common radiologic findings for IPNB-L include intraductal mass and bile duct dilatation, which are also found in other liver tumors [1617]. Both IPNB-L with HGIEN and that with carcinoma are considered to be malignant IPNB-L and the same surgical strategy is applied in patients with these 2 pathologic findings. In the present study, IPNB-L with HGIEN/carcinoma accounted for 81.2% of the study cases, whereas IPNB-L with low-grade neoplasia occupied 18.8%. It is difficult to discern benign from malignant IPNB-L through CT and MRI-based preoperative imaging findings.
FDG-PET is reported to be useful for identifying malignant IPNB [8]. The majority of HGIEN/carcinoma IPNB-Ls showed a higher SUVmax than IPNB-L with low-grade neoplasia in the present study, as observed in other organ malignancies [181920]. IPNB is considered to be the biliary counterpart of intraductal papillary mucinous neoplasm (IPMN) of the pancreas. Similar to IPNB, IPMN presents at various histopathological stages ranging from benign to malignant, thus preoperative differentiation between benign and malignant IPMN is also important to determine the treatment strategy. It was reported that the SUVmax in patients with malignant IPMN was significantly higher than that in patients with benign IPMN [20].
A reliable SUVmax cutoff for predicting IPNB-L of HGIEN and carcinoma has not yet been established. In the present study, the diagnostic sensitivity and specificity of an FDG-PET SUVmax cutoff of 3.0 were 84.2% and 47.4% respectively in all 101 patients; 73.2% and 47.4% respectively with 19 patients with low-grade neoplasia IPNB-L and early 41 patients with HGIEN/carcinoma IPNB-L; and 92.9% and 47.4%, respectively, with 19 patients with low-grade neoplasia IPNB-L and late 41 patients with HGIEN/carcinoma IPNB-L. Interestingly, the late subgroup patients taken FDG-PET and surgery after 2014 showed much higher sensitivity.
A Japanese study has proposed an SUVmax cutoff of 4.5 for predicting IPNB with invasive carcinoma, with accuracy, sensitivity, and specificity of 94.5%, 76.0%, and 100%, respectively [8]. However, when applying this cutoff value to our study patients for predicting IPNB-L of HGIEN and carcinoma, the sensitivity and specificity were only 54.9% and 54.9%, respectively, which was below the level of reliability needed in clinical practice.
In the present study, we developed a SUVmax cutoff of 3.0 through a ROC curve analysis. Its area under the curve was 0.674 with a sensitivity of 84.2% and a specificity of 47.4%. Additional internal validation study with recent patients showed enhanced sensitivity up to 92.9%. The positive predictive values found using precision-recall curve analysis to be well maintained at over 80% along a wide range of sensitivity changes. The proportion of malignant IPNB-L was statistically higher in patients with SUVmax than those with SUVmax of ≤3.0. We suggest therefore that a SUVmax cutoff of 3.0 is a useful value for predicting IPNB-L with HGIEN or carcinoma. However, when we evaluated the prognostic impact of FDG-PET SUVmax cutoff of 3.0, it was not found to be associated with the postresection prognosis.
External validation of FDG-PET SUVmax cutoff is essential to establish the truly reliable cutoff to predict malignant IPNB-L. To the best of our knowledge, there are only 2 cutoff values on the FDG-PET SUVmax cutoff; they are 4.5 from above mentioned Japanese study [8] and 3.0 from the present study. Considering that IPNBs share similar morphological, clinicopathological, and biological features with IPMNs of the pancreas, IPNB is considered the biliary counterpart of pancreatic IPMN. It was reported that the FDG-PET SUVmax in patients with malignant IPMN was significantly higher than in patients with benign IPMN [202122232425].
The present study had some limitations of note. This study was a retrospective study using single-center data and the sample size was relatively small. High-volume multicenter studies will be needed to validate the results of the present study.
In conclusion, IPNB-L is a rare type of biliary neoplasm that encompasses a histological spectrum ranging from low-grade intraepithelial neoplasia to invasive carcinoma. An FDG-PET SUVmax cutoff of 3.0 appears to be effective for discerning HGIEN/carcinoma from low-grade neoplasia IPNB-L, which will assist with the surgical strategy for IPNB-L.
References
1. Nakanuma Y, Sato Y, Harada K, Sasaki M, Xu J, Ikeda H. Pathological classification of intrahepatic cholangiocarcinoma based on a new concept. World J Hepatol. 2010; 2:419–427. PMID: 21191517.

2. Jang KT, Hong SM, Lee KT, Lee JG, Choi SH, Heo JS, et al. Intraductal papillary neoplasm of the bile duct associated with Clonorchis sinensis infection. Virchows Arch. 2008; 453:589–598. PMID: 18855009.

3. Itatsu K, Zen Y, Ohira S, Ishikawa A, Sato Y, Harada K, et al. Immunohistochemical analysis of the progression of f lat and papillary preneoplastic lesions in intrahepatic cholangiocarcinogenesis in hepatolithiasis. Liver Int. 2007; 27:1174–1184. PMID: 17919228.

4. Zen Y, Fujii T, Itatsu K, Nakamura K, Minato H, Kasashima S, et al. Biliary papillary tumors share pathological features with intraductal papillary mucinous neoplasm of the pancreas. Hepatology. 2006; 44:1333–1343. PMID: 17058219.

5. Nakanuma Y. A novel approach to biliary tract pathology based on similarities to pancreatic counterparts: is the biliary tract an incomplete pancreas? Pathol Int. 2010; 60:419–429. PMID: 20518896.

6. World Health Organization (WHO) Classification of Tumours Editorial Board. WHO classification of tumours, digestive system tumours. 5th ed. International Agency for Research on Cancer: Lyon, France;2019. p. 279–282.
7. Liu Y, Zhong X, Yan L, Zheng J, Liu Z, Liang C. Diagnostic performance of CT and MRI in distinguishing intraductal papillary neoplasm of the bile duct from cholangiocarcinoma with intraductal papillary growth. Eur Radiol. 2015; 25:1967–1974. PMID: 25716939.

8. Ikeno Y, Seo S, Yamamoto G, Nakamoto Y, Uemoto Y, Fuji H, et al. Usefulness of preoperative 18F-FDG-PET in detecting invasive intraductal papillary neoplasm of the bile duct. Anticancer Res. 2018; 38:3677–3682. PMID: 29848727.

9. Hwang S, Lee YJ, Song GW, Park KM, Kim KH, Ahn CS, et al. Prognostic impact of tumor growth type on 7th AJCC staging system for intrahepatic cholangiocarcinoma: a single-center experience of 659 cases. J Gastrointest Surg. 2015; 19:1291–1304. PMID: 25820487.

10. Wu SD, Lu CD, Lu CJ, Huang J, Zhou J. Mucin-producing intrahepatic biliary papillomatosis. Surg Today. 2010; 40:845–850. PMID: 20740348.

11. Nakanuma Y, Zen Y, Harada K, Ikeda H, Sato Y, Uehara T, et al. Tumorigenesis and phenotypic characteristics of mucin-producing bile duct tumors: an immunohistochemical approach. J Hepatobiliary Pancreat Sci. 2010; 17:211–222. PMID: 19680592.

12. Yeh CN, Jan YY, Yeh TS, Hwang TL, Chen MF. Hepatic resection of the intraductal papillary type of peripheral cholangiocarcinoma. Ann Surg Oncol. 2004; 11:606–611. PMID: 15172934.

13. Onoe S, Shimoyama Y, Ebata T, Yokoyama Y, Igami T, Sugawara G, et al. Prognostic delineation of papillary cholangiocarcinoma based on the invasive proportion: a single-institution study with 184 patients. Surgery. 2014; 155:280–291. PMID: 24287144.

14. Rocha FG, Lee H, Katabi N, DeMatteo RP, Fong Y, D’Angelica MI, et al. Intraductal papillary neoplasm of the bile duct: a biliary equivalent to intraductal papillary mucinous neoplasm of the pancreas? Hepatology. 2012; 56:1352–1360. PMID: 22504729.

15. Barton JG, Barrett DA, Maricevich MA, Schnelldorfer T, Wood CM, Smyrk TC, et al. Intraductal papillary mucinous neoplasm of the biliary tract: a real disease? HPB (Oxford). 2009; 11:684–691. PMID: 20495637.

16. Park HJ, Kim SY, Kim HJ, Lee SS, Hong GS, Byun JH, et al. Intraductal papillary neoplasm of the bile duct: clinical, imaging, and pathologic features. AJR Am J Roentgenol. 2018; 211:67–75. PMID: 29629808.

17. Nakanuma Y, Sato Y, Ojima H, Kanai Y, Aishima S, Yamamoto M, et al. Clinicopathological characterization of so-called “cholangiocarcinoma with intraductal papillary growth” with respect to “intraductal papillary neoplasm of bile duct (IPNB)”. Int J Clin Exp Pathol. 2014; 7:3112–3122. PMID: 25031730.
18. Jadvar H, Alavi A, Gambhir SS. 18F-FDG uptake in lung, breast, and colon cancers: molecular biology correlates and disease characterization. J Nucl Med. 2009; 50:1820–1827. PMID: 19837767.

19. Seo S, Hatano E, Higashi T, Hara T, Tada M, Tamaki N, et al. Fluorine-18 fluorodeoxyglucose positron emission tomography predicts tumor differentiation, P-glycoprotein expression, and outcome after resection in hepatocellular carcinoma. Clin Cancer Res. 2007; 13(2 Pt 1):427–433. PMID: 17255262.

20. Tomimaru Y, Takeda Y, Tatsumi M, Kim T, Kobayashi S, Marubashi S, et al. Utility of 2-[18F] fluoro-2-deoxy-D-glucose positron emission tomography in differential diagnosis of benign and malignant intraductal papillary-mucinous neoplasm of the pancreas. Oncol Rep. 2010; 24:613–620. PMID: 20664965.

21. Yamashita YI, Okabe H, Hayashi H, Imai K, Nakagawa S, Nakao Y, et al. Usefulness of 18-FDG PET/CT in detecting malignancy in intraductal papillary mucinous neoplasms of the pancreas. Anticancer Res. 2019; 39:2493–2499. PMID: 31092444.

22. Bertagna F, Treglia G, Baiocchi GL, Giubbini R. F18-FDG-PET/CT for evaluation of intraductal papillary mucinous neoplasms (IPMN): a review of the literature. Jpn J Radiol. 2013; 31:229–236. PMID: 23315020.

23. Serafini S, Sperti C, Brazzale AR, Cecchin D, Zucchetta P, Pierobon ES, et al. The role of positron emission tomography in clinical management of intraductal papillary mucinous neoplasms of the pancreas. Cancers (Basel). 2020; 12:807.

24. Huo L, Feng F, Liao Q, Jin Z, Li F, Zhao Y. Intraductal papillary mucinous neoplasm of the pancreas with high malignant potential on FDG PET/MRI. Clin Nucl Med. 2016; 41:989–990. PMID: 27764041.

25. Roch AM, Barron MR, Tann M, Sandrasegar K, Hannaford KN, Ceppa EP, et al. Does PET with CT have clinical utility in the management of patients with intraductal papillary mucinous neoplasm? J Am Coll Surg. 2015; 221:48–56. PMID: 26095551.

Fig. 1
Comparison of tumor recurrence (A) and overall patient survival (B) curves according to the histological tumor grade. IPNB-L, intraductal papillary neoplasm of the bile duct of the liver.
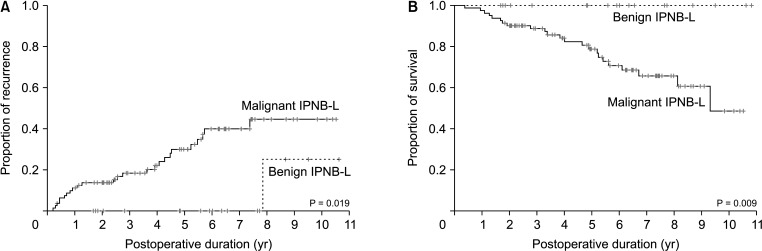
Fig. 2
Distribution of fluorodeoxyglucose-PET maximal standardized uptake values (SUVmax) according to the histological tumor grade. IPNB-L, intraductal papillary neoplasm of the bile duct of the liver.
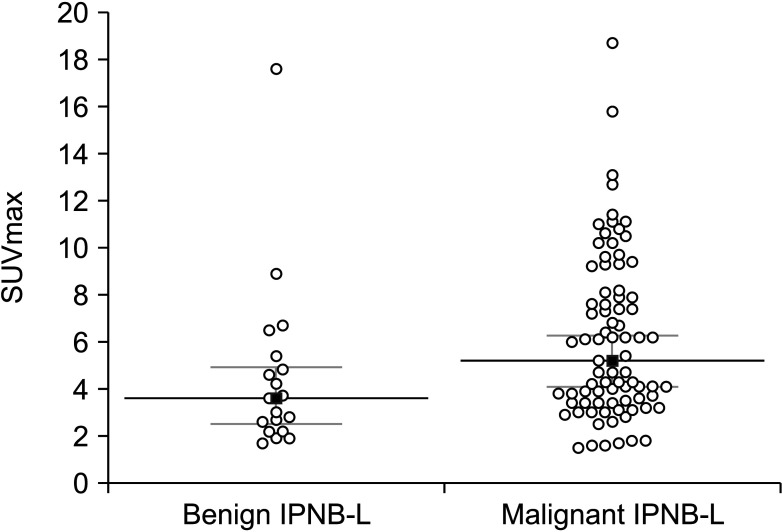
Fig. 3
Receiver operating characteristic curve analysis for predicting intraductal papillary neoplasms of the bile duct of the liver with high-grade intraepithelial neoplasia and invasive carcinoma.
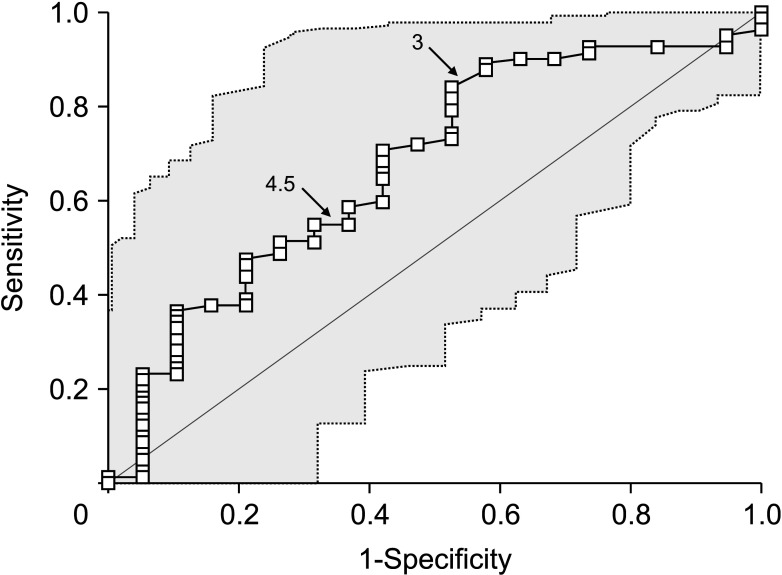
Fig. 4
Validation using precision-recall curve analysis matched with receiver operating characteristic curve analysis for intraductal papillary neoplasms of the bile duct of the liver with high-grade intraepithelial neoplasia and invasive carcinoma. PPV, positive predictive value.
Fig. 5
Comparison of tumor recurrence (A) and overall patient survival (B) curves according to a fluorodeoxyglucose-PET maximal standardized uptake value (SUVmax) cutoff of 3.0 in patients with intraductal papillary neoplasms of the bile duct of the liver with high-grade intraepithelial neoplasia and invasive carcinoma.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download